��Ŀ����
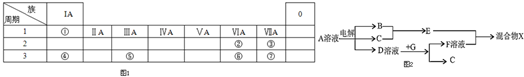
17��A��B��C��D��Ϊ��ѧ��ѧ�г����ĵ��ʻ������֮��Ĺ�ϵ��ͼ1��ʾ�����ֲ�������ȥ����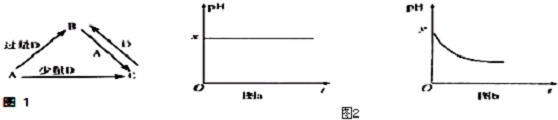
��1����A��BΪ�Σ�DΪǿ�A��ˮ��Һ�����ԣ���Ӧ C+D��B�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O
��2����AΪ�������ʣ�D��ijǿ���ϡ��Һ����ӦB+A��C�����ӷ���ʽΪ2Fe3++Fe=3Fe2+
��3����AΪǿ�DΪ��̬���������ʱ����B��ˮ��Һ¶���ڿ����У���pH��ʱ��t�仯������ͼ2��a��ͼb��ʾ��������D���ܽ��ˮ�Ļӷ�����
����ͼa������ʵ����D�ĵ���ʽΪ
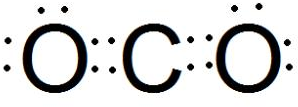 ��
������ͼb������ʵ����ͼb��y��7��B����ɫ��ӦΪ��ɫ����C��Һ�и�����Ũ���ɴ�С��˳����c��Na+����c��SO3-����c��OH-����c��HSO3-����c��H+����
���� ��1����A��BΪ�Σ�DΪǿ�A��ˮ��Һ�����ԣ�A�Ͳ�ͬ���ļӦ���ɲ�ͬ�����ʣ���A�����Σ����κ���ǿ�Ӧ����ƫ�����Σ��������Ӧ���������������������ӷ���ʽ����д������д��
��2����AΪ�������ʣ�D��ijǿ���ϡ��Һ��A��D��Ӧʱ��A������ͬ�����ﲻͬ����A�DZ�۽�������A�dz���������Ϊ�����������ӷ���ʽ����д������д��
��3������AΪǿ�DΪ��̬���������ʱ����B��ˮ��Һ¶���ڿ����У���pH��ʱ��t�仯��ͼ����ͼa������ʵ��˵��������������Ϊ�ǻ�ԭ����������������ε�����ȷ����Һ��pHֵ��С��
����ͼb������ʵ��˵��������������Ϊ��ԭ�����������ͼb��y��7��B����ɫ��ӦΪ��ɫ��˵��������Ԫ�أ���B�����������ƣ�A���������ƣ�C���������ƣ�������������ǿ�������Σ�����ˮ���ʹ��Һ�����ԣ�
��� �⣺��1����A��BΪ�Σ�DΪǿ�A��ˮ��Һ�����ԣ�A�Ͳ�ͬ���ļӦ���ɲ�ͬ�����ʣ���A�����Σ����κ���ǿ�Ӧ����ƫ�����Σ��������Ӧ����������������Ӧ C+D��B�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��2����AΪ�������ʣ�D��ijǿ���ϡ��Һ��A��D��Ӧʱ��A������ͬ�����ﲻͬ����A�DZ�۽�������A�dz�����������AΪ����B�����Σ�C�������Σ������Ӻ�����Ӧ�����������ӣ����ӷ�Ӧ����ʽΪ��2Fe3++Fe=3Fe2+��
�ʴ�Ϊ��2Fe3++Fe=3Fe2+��
��3������AΪǿ�DΪ��̬�������������ʱ����B��ˮ��Һ¶���ڿ����У���ͼa������ʵ��˵��������������Ϊ�ǻ�ԭ�������������DΪ������̼����D�ĵ���ʽΪ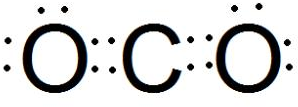 ���ʴ�Ϊ��
���ʴ�Ϊ��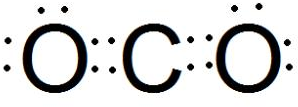 ��
��
����ͼb������ʵ��˵��������������Ϊ��ԭ�����������ͼb��y��7��B����ɫ��ӦΪ��ɫ��˵��������Ԫ�أ���B�����������ƣ�A���������ƣ�C���������ƣ�����������ǿ���������Σ������������ˮ��������������������������ӣ������Ӳ�ˮ�⣬ˮҲ����������������ӣ�������Һ������Ũ�ȴ�С˳���� c��Na+����c��SO3-����c��OH-����c��HSO3-����c��H+����
�ʴ�Ϊ��cc��Na+����c��SO3-����c��OH-����c��HSO3-����c��H+����
���� ���⿼��������Ũ�ȴ�С�ıȽϡ�Ԫ�ػ���������ʵȣ�ע��Ԫ�ػ������������Ӧ�ǽⱾ��ؼ����ѶȲ���

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�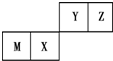
| A�� | ԭ�Ӱ뾶Z��M | |
| B�� | Y������������Ӧˮ��������Ա�X���� | |
| C�� | X�������̬�⻯������ȶ��Ա�Z��С | |
| D�� | Zλ��Ԫ�����ڱ��е�2���ڵڢ�A�� |
| A�� | 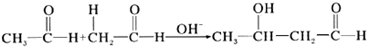 | |
| B�� | CH2=CH-CH=CH2+2H2$��_{��}^{����}$CH3-CH2-CH-CH3 | |
| C�� | 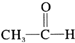 +H2$��_{��}^{����}$CH3-CH-OH +H2$��_{��}^{����}$CH3-CH-OH | |
| D�� | CH3-CH2Br+NaOH$��_{��}^{H_{2}O}$CH3-CH2OH+NaBr |
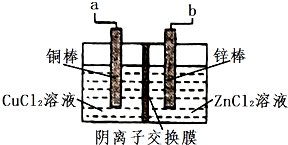 һ��ʹ�������ӽ���Ĥ��ֻҲ��������ͨ������ͭп��ؽṹ�ṹ��ͼ�����ѡ������������ȷ����������ǣ�������
һ��ʹ�������ӽ���Ĥ��ֻҲ��������ͨ������ͭп��ؽṹ�ṹ��ͼ�����ѡ������������ȷ����������ǣ�������| ѡ�� | ���������� | ���������� |
| A | ��طŵ�ʱ��ѧ��ת��Ϊ���� | Zn��s��+Cu2+��aq��=Zn2++Cu��s����H��0 |
| B | �õ�س��ʱͭ����ϸ | ���缫����Һ��ɫ������ |
| C | �õ�طŵ�ʱͭ���ǵ������ | Cl-ͨ������Ĥ����ͭ���������ң�п�������ƶ� |
| D | �õ�س��ʱa�ӵ�Դ���� | �缫��ӦCu2++2e-=Cu |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��ϵͳ�������������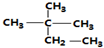 ��������Ϊ2-��-2-�һ����� ��������Ϊ2-��-2-�һ����� | |
| B�� | 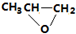 ��CO2ת��Ϊ ��CO2ת��Ϊ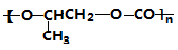 �ķ�Ӧ������ɫ��ѧ��ԭ�� �ķ�Ӧ������ɫ��ѧ��ԭ�� | |
| C�� | ������������ͭ����Һ������ʧȥ��ǩ���Ҵ�����ȩ��������ƿ��ɫ��Һ | |
| D�� | �Ҵ������ѻ�Ϊͬ���칹�壬�е��Ҵ������Ѹ� |
| A�� | ��������ͭ�ֱ�������ϡ�����Ũ�����ַ�Ӧ��������������ʵ�����ͬ | |
| B�� | �����������ֱ����������������ַ�Ӧ���������ʵ����ʵ�����ͬ | |
| C�� | �����������ֱ�����������������Һ�������ַ�Ӧ��������������һ����ͬ | |
| D�� | �������������ֱ��������������ƺ����Ƴ�ַ�Ӧ��ת�Ƶĵ�������ͬ |
| A�� | ���õķ�ɢϵ���ڽ��壬�ɷ��������ЧӦ | |
| B�� | ���õķ�ɢϵ�У���ɢ�ʵ���Ҫ�ɷ�ΪFeO | |
| C�� | �÷�ɢϵ���е�Ӿʵ��ʱ��������Χ��ɫ���� | |
| D�� | ��������ˮ��Һ�еμ�Ũ��ˮ������������ |
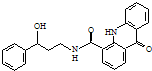
| A�� | ��X�ķ���ʽΪC23H25N2O3 | |
| B�� | ÿ��X�����к���2������̼ԭ�� | |
| C�� | 1 mol X�������9 mol H2�����ӳɷ�Ӧ | |
| D�� | X�ܷ���ˮ�⡢��������ȥ��Ӧ |