��Ŀ����
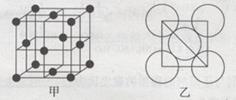
ԭ��������������Ķ�����Ԫ��a��b��c��d��e�У�a������������Ϊ���������Ķ�����b��d��A2B���⻯���ΪV�η��ӣ�c�ģ�1�����ӱ�e�ģ�1��������8�����ӡ�
�ش��������⣺
(1)Ԫ��aΪ ��cΪ ��
(2)����ЩԪ���γɵ�˫ԭ�ӷ���Ϊ ��
(3)����ЩԪ���γɵ���ԭ�ӷ����У����ӵĿռ�ṹ����ֱ���ε��� ����ֱ���ε��� ��(д2��)
(4)��ЩԪ�صĵ��ʻ��������γɵ�AB�ͻ������У��侧����������ԭ�Ӿ������ �����Ӿ������ ������������� �����Ӿ������ ��(ÿ����һ��)
(5)Ԫ��a��b�γɵ�һ�ֻ�������c��b�γɵ�һ�ֻ�������ķ�Ӧ�����ڷ�������У��÷�Ӧ�Ļ�ѧ����ʽΪ ��
�ش��������⣺
(1)Ԫ��aΪ ��cΪ ��
(2)����ЩԪ���γɵ�˫ԭ�ӷ���Ϊ ��
(3)����ЩԪ���γɵ���ԭ�ӷ����У����ӵĿռ�ṹ����ֱ���ε��� ����ֱ���ε��� ��(д2��)
(4)��ЩԪ�صĵ��ʻ��������γɵ�AB�ͻ������У��侧����������ԭ�Ӿ������ �����Ӿ������ ������������� �����Ӿ������ ��(ÿ����һ��)
(5)Ԫ��a��b�γɵ�һ�ֻ�������c��b�γɵ�һ�ֻ�������ķ�Ӧ�����ڷ�������У��÷�Ӧ�Ļ�ѧ����ʽΪ ��
(1)̼ ��
(2)CO O2 Cl2
(3)CO2��CS2 SO2��O3��SCl2��Cl2O�������
(4)���ʯ NaCl Na CO(O2��Cl2)
(5)2CO2��2Na2O2=2Na2CO3��O2
(2)CO O2 Cl2
(3)CO2��CS2 SO2��O3��SCl2��Cl2O�������
(4)���ʯ NaCl Na CO(O2��Cl2)
(5)2CO2��2Na2O2=2Na2CO3��O2
a������������Ϊ���������Ķ�����������Ԫ������̼( )����(
)����( )����Ϊ�����������Ԫ�أ��������������Ԫ��Ҫ������a��̼��������Ԫ���γ�A2B��������ΪV�η��ӵ�Ϊ������bΪ����dΪ��c�ģ�1�����ӱ�e�ģ�1��������8�����ӣ�cΪ�ƣ�eΪ�ȡ�
)����Ϊ�����������Ԫ�أ��������������Ԫ��Ҫ������a��̼��������Ԫ���γ�A2B��������ΪV�η��ӵ�Ϊ������bΪ����dΪ��c�ģ�1�����ӱ�e�ģ�1��������8�����ӣ�cΪ�ƣ�eΪ�ȡ�
(2)����ЩԪ���γɵ�˫ԭ�ӷ�����CO��O2��Cl2��
(3)��ЩԪ���γɵ���ԭ�ӷ����У�ֱ���ε���CO2��CS2����ֱ���ε��У�SO2��O3��SCl2��Cl2O�ȡ�
(4)��ЩԪ�صĵ�������ԭ�Ӿ�����ǽ��ʯ��O2��Cl2��Ϊ���Ӿ��壬��Ϊ�������壬AB�ͻ�������NaCl��CO��NaClΪ���Ӿ��壬COΪ���Ӿ��塣
(5)a(̼)��b(��)�γɵ�CO2����C(��)��b(��)�γɵ�Na2O2������Ӧ��2Na2O2��2CO2=2Na2CO3��O2��
 )����(
)����( )����Ϊ�����������Ԫ�أ��������������Ԫ��Ҫ������a��̼��������Ԫ���γ�A2B��������ΪV�η��ӵ�Ϊ������bΪ����dΪ��c�ģ�1�����ӱ�e�ģ�1��������8�����ӣ�cΪ�ƣ�eΪ�ȡ�
)����Ϊ�����������Ԫ�أ��������������Ԫ��Ҫ������a��̼��������Ԫ���γ�A2B��������ΪV�η��ӵ�Ϊ������bΪ����dΪ��c�ģ�1�����ӱ�e�ģ�1��������8�����ӣ�cΪ�ƣ�eΪ�ȡ�(2)����ЩԪ���γɵ�˫ԭ�ӷ�����CO��O2��Cl2��
(3)��ЩԪ���γɵ���ԭ�ӷ����У�ֱ���ε���CO2��CS2����ֱ���ε��У�SO2��O3��SCl2��Cl2O�ȡ�
(4)��ЩԪ�صĵ�������ԭ�Ӿ�����ǽ��ʯ��O2��Cl2��Ϊ���Ӿ��壬��Ϊ�������壬AB�ͻ�������NaCl��CO��NaClΪ���Ӿ��壬COΪ���Ӿ��塣
(5)a(̼)��b(��)�γɵ�CO2����C(��)��b(��)�γɵ�Na2O2������Ӧ��2Na2O2��2CO2=2Na2CO3��O2��

��ϰ��ϵ�д�
�����Ŀ









 �������ʵ���Ϊ mol��
�������ʵ���Ϊ mol��