��Ŀ����
ͭ���ʼ��仯�����ںܶ������ж�����Ҫ����;����ش��������⣺
(1)��ϸͭ�ۿ�����������ϡ������ȣ����Ʊ��������£�

��NH4CuSO3�н��������ӵĺ�������Ų�ʽΪ______��N��O��S����Ԫ�صĵ�һ�����ܴ�С˳��Ϊ__________________(��Ԫ�ط���)��
��SO42-�Ŀռ乹��Ϊ________��
(2)ijѧ��������ͭ��Һ�백ˮ����һ��ʵ�飬CuSO4��Һ�мӰ�ˮ������ɫ�������ټӰ�ˮ�����ܽ⣬�õ�����ɫ����Һ����������Һ�м���һ�����Ҵ�������[Cu(NH3)4]SO4��H2O���壬����ͼ����Ҵ������������ԭ��_________���ڸþ����д��ڵĻ�ѧ����������_________��
(3)�Ⱥͼ��벻ͬ��̬��ͭ���������ֻ�����������Ӿ�Ϊ�������ṹ(��ͼ��ʾ)��aλ����Clԭ�ӵ��ӻ��������Ϊ________����֪����һ�ֻ�����Ļ�ѧʽΪKCuCl3������һ�ֻ�����Ļ�ѧʽΪ____________��

�þ����X�������䷨���Բ�ð����ӵ�������ֵ���Խ���ͭ�IJⶨ�õ����½����ͭ����Ϊ�����������ܶѻ����߳�Ϊ361 pm����֪ͭ���ܶ�Ϊ9.00 g��cm��3����ͭԭ�ӵ�ֱ��ԼΪ__________pm�������ӵ�������ֵΪ________[��֪Ar(Cu)��63.5]��
(1)��ϸͭ�ۿ�����������ϡ������ȣ����Ʊ��������£�

��NH4CuSO3�н��������ӵĺ�������Ų�ʽΪ______��N��O��S����Ԫ�صĵ�һ�����ܴ�С˳��Ϊ__________________(��Ԫ�ط���)��
��SO42-�Ŀռ乹��Ϊ________��
(2)ijѧ��������ͭ��Һ�백ˮ����һ��ʵ�飬CuSO4��Һ�мӰ�ˮ������ɫ�������ټӰ�ˮ�����ܽ⣬�õ�����ɫ����Һ����������Һ�м���һ�����Ҵ�������[Cu(NH3)4]SO4��H2O���壬����ͼ����Ҵ������������ԭ��_________���ڸþ����д��ڵĻ�ѧ����������_________��
(3)�Ⱥͼ��벻ͬ��̬��ͭ���������ֻ�����������Ӿ�Ϊ�������ṹ(��ͼ��ʾ)��aλ����Clԭ�ӵ��ӻ��������Ϊ________����֪����һ�ֻ�����Ļ�ѧʽΪKCuCl3������һ�ֻ�����Ļ�ѧʽΪ____________��

�þ����X�������䷨���Բ�ð����ӵ�������ֵ���Խ���ͭ�IJⶨ�õ����½����ͭ����Ϊ�����������ܶѻ����߳�Ϊ361 pm����֪ͭ���ܶ�Ϊ9.00 g��cm��3����ͭԭ�ӵ�ֱ��ԼΪ__________pm�������ӵ�������ֵΪ________[��֪Ar(Cu)��63.5]��
(1)��1s22s22p63s23p63d10(��[Ar]3d10)��N>O>S��
������������
(2)�Ҵ����Ӽ��Ա�ˮ���Ӽ������������Ҵ������ܼ��ļ��ԣ��Ӷ���С���ʵ��ܽ�ȣ����Ӽ�����λ�������ۼ�
(3)sp3�ӻ� ��K2CuCl3
(4)��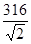 pm ��6.01��1023
pm ��6.01��1023
������������
(2)�Ҵ����Ӽ��Ա�ˮ���Ӽ������������Ҵ������ܼ��ļ��ԣ��Ӷ���С���ʵ��ܽ�ȣ����Ӽ�����λ�������ۼ�
(3)sp3�ӻ� ��K2CuCl3
(4)��
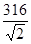 pm ��6.01��1023
pm ��6.01��1023(1)NH4CuSO3�е�����������Cu+�����ĺ�������Ų��ǣ�1s22s22p63s23p63d10(��[Ar]3d10)������ͬһ���ڵ�һ�����ܱ仯���ɼ��ڢ�A����A����֪����һ�����ܴ���˳��Ϊ��N>O>S��SO42-��AB4�ͣ�����ռ乹�����������壻
(2)�Ҵ����Ӽ��Ա�ˮ���Ӽ������������Ҵ������ܼ��ļ��ԣ��Ӷ���С���ʵ��ܽ�ȡ��þ����Ļ�ѧ�������Ӽ�����λ�����ۼ���
(3)aλ����Clԭ�ӳ�2������������2�Թ¶Ե��ӣ��ӻ������Ϊ4���ӻ��������Ϊsp3��һ�ֻ�����Ļ�ѧʽΪKCuCl3������ͭԪ��Ϊ+2�ۣ�����һ�ֻ�������ͭΪ+1�ۣ�CuCl3ԭ���ŵĻ��ϼ�Ϊ-2���仯ѧʽΪ��K2CuCl3��
(4)��ͭԭ�ӵ�ֱ����d��������������������߳�(361)2=2d2������ֱ����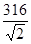 pm�������������V=(361��10-10)3cm3����������4��ͭԭ�ӣ�����������m=4/NAmol��63.5g/mol����9.00 g��cm��3=m/V��NA=6.01��1023��
pm�������������V=(361��10-10)3cm3����������4��ͭԭ�ӣ�����������m=4/NAmol��63.5g/mol����9.00 g��cm��3=m/V��NA=6.01��1023��
(2)�Ҵ����Ӽ��Ա�ˮ���Ӽ������������Ҵ������ܼ��ļ��ԣ��Ӷ���С���ʵ��ܽ�ȡ��þ����Ļ�ѧ�������Ӽ�����λ�����ۼ���
(3)aλ����Clԭ�ӳ�2������������2�Թ¶Ե��ӣ��ӻ������Ϊ4���ӻ��������Ϊsp3��һ�ֻ�����Ļ�ѧʽΪKCuCl3������ͭԪ��Ϊ+2�ۣ�����һ�ֻ�������ͭΪ+1�ۣ�CuCl3ԭ���ŵĻ��ϼ�Ϊ-2���仯ѧʽΪ��K2CuCl3��
(4)��ͭԭ�ӵ�ֱ����d��������������������߳�(361)2=2d2������ֱ����
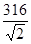 pm�������������V=(361��10-10)3cm3����������4��ͭԭ�ӣ�����������m=4/NAmol��63.5g/mol����9.00 g��cm��3=m/V��NA=6.01��1023��
pm�������������V=(361��10-10)3cm3����������4��ͭԭ�ӣ�����������m=4/NAmol��63.5g/mol����9.00 g��cm��3=m/V��NA=6.01��1023��
��ϰ��ϵ�д�
�����Ŀ


 ������������������γ���������[ Co(NO2)6]3������������������K�����䷴Ӧ���£�3K����[Co(NO2)6]3��=K3[Co(NO2)6]��(����ɫ)��
������������������γ���������[ Co(NO2)6]3������������������K�����䷴Ӧ���£�3K����[Co(NO2)6]3��=K3[Co(NO2)6]��(����ɫ)��
 ������ĿΪ ��
������ĿΪ ��


