��Ŀ����
13��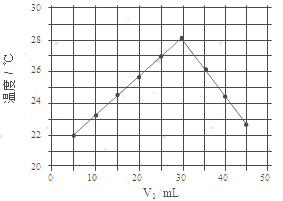 ˮ�����ǰ��������������������������Ҫ��Դ��Ҳ�ǻ�ѧ��Ӧ�е���Ҫ���ʣ�
ˮ�����ǰ��������������������������Ҫ��Դ��Ҳ�ǻ�ѧ��Ӧ�е���Ҫ���ʣ���1��ijѧϰ��ȤС������H2SO4��Һ�� NaOH��Һ��Ӧ�����к��ȣ���V1 mL 0.4mol/L ��H2SO4��Һ��V2 mLδ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ��������¼��Һ�¶ȣ�ʵ������ͼ��ʾ��ʵ����ʼ�ձ���V1+V2=50mL����
������NaOH��Һ���ʵ���Ũ��Ϊ1.2 mol/L
��ʵ�����к��ȡ�H=-57.3kJ•mol-1��д����H2SO4��Һ�� NaOH��Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ$\frac{1}{2}$H2SO4��aq��+NaOH��aq��=$\frac{1}{2}$Na2SO4��aq��+2H2O��l����H=-57.3kJ/mol��
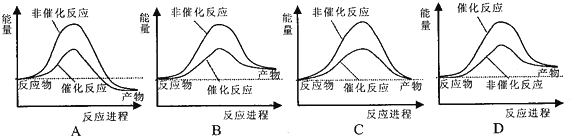
��2���ݱ�������ѧ�ҿ�����������̫���ֽܷ�ˮ�����ʹ����������й�ˮ�ֽ���̵������仯ʾ��ͼ��ȷ����B
��3��������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮
��֪��
CH4��g��+H2O��g���TCO��g��+3H2��g����H=+206.2kJ•mol-1
CH4��g��+CO2��g���T2CO��g��+2H2��g����H=-247.4kJ•mol-1
�Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ�������������д��CH4��g����H2O��g����Ӧ����CO2��g����H2��g�����Ȼ�ѧ����ʽCH4��g��+2H2O��g���TCO2��g��+4H2��g����H=+659.8kmol-1��
��4������ˮ�����ֽ�Ҳ�����Ƶ��������±�Ϊ���ֳ�����ѧ���ļ������ݣ�
| ���� | H-H | O=O | H-O |
| ����/��kJ•mol-1�� | 436 | 498 | 464 |
���� ��1������ͼ��֪��ǡ�÷�Ӧʱ�μӷ�Ӧ��������Һ�������30mL����V1+V2=50mL��֪�����ĵ�����������Һ�����Ϊ20mL���Դ����NaOH��Һ��Ũ�ȣ�
�ڸ����к��ȸ�����кͷ�Ӧ����1molҺ̬ˮʱ�ų�57.3kJ��������д�Ȼ�ѧ����ʽ��
��2��ˮ�ķֽ�Ϊ���ȷ�Ӧ����Ӧ���������С�������������������Ӧ�м�������ή�ͻ�ܣ��ı䷴Ӧ�����ʣ�����Ӧ�Ȳ��ı䣻
��3�����ø�˹���ɽ�𣬴Ӵ���Ӧ����������Ӧ���������������Ӧ�е�λ�ã�ͨ����Ӽ��ɵã�
��4���ȸ���2H2O��g��=2H2��g��+O2��g����ϻ�ѧ���ܼ����H��Ȼ����ݡ�H�Ƶ�2gH2����Ҫ���յ�������ע��1molˮ�к���2molH-O����
��� �⣺��1����ǡ�÷�Ӧʱ�μӷ�Ӧ��������Һ�������30mL����V1+V2=50mL�����ĵ�����������Һ�����Ϊ20mL����ǡ�÷�Ӧʱ����������Һ�����ʵ����ʵ���Ϊn��
H2SO4 +2NaOH�TNa2SO4 +2H2O
1 2
0.4mol•L-1��0.03L n
��n=0.4mol•L-1��0.03L��2=0.024mol��
����Ũ��C=$\frac{0.024mol}{0.02L}$=1.2mol/L��
�ʴ�Ϊ��1.2��
��ʵ�����к��ȡ�H=-57.3kJ•mol-1��ϡǿ�ᡢϡǿ�Ӧ����1molҺ̬ˮʱ�ų�57.3kJ��������ϡ���������������ϡ��Һ����ǿ���ǿ���ϡ��Һ����Ӧ���Ȼ�ѧ����ʽΪ��$\frac{1}{2}$H2SO4��aq��+NaOH��aq��=$\frac{1}{2}$Na2SO4��aq��+2H2O��l����H=-57.3kJ/mol��
�ʴ�Ϊ��$\frac{1}{2}$H2SO4��aq��+NaOH��aq��=$\frac{1}{2}$Na2SO4��aq��+2H2O��l����H=-57.3kJ/mol��
��2��A��ˮ�ķֽ�Ϊ���ȷ�Ӧ����Ӧ���������С�������������������ͼ��������A����
B�����������Ӧ�Ȳ��䣬����ͼ����Ϸ�Ӧ���������С�������������������B��ȷ��
C����ѧ��Ӧһ�������������仯����Ӧ����������������������������ȣ���C����
D������������ͷ�Ӧ�Ļ�ܣ�ͼ���ϣ���D����
��ѡB��
��3����CH4��g��+H2O��g���TCO��g��+3H2��g����H=+206.2kmol-1
��CH4��g��+CO2��g���T2CO��g��+2H2��g����H=-247.4kJmol-1
�ݸ�˹���ɣ��١�2-�ڵã�CH4��g��+2H2O��g���TCO2��g��+4H2��g����H=+659.8kmol-1��
�ʴ�Ϊ��CH4��g��+2H2O��g���TCO2��g��+4H2��g����H=+659.80kmol-1��
��4��1molˮ�к���2molH-O������H=��Ӧ����ܼ���-��������ܼ��ܣ�����2H2O��g��=2H2��g��+O2��g����H=4��464kJ/mol-2��436kJ/mol-498kJ/mol=+486kJ/mol��2gH2�����ʵ���Ϊ$\frac{2g}{2g/mol}$=1mol����������ˮ�����ֽ��Ƶ�2gH2����Ҫ���յ�����$\frac{486KJ}{2}$=243KJ���ʴ�Ϊ��243��
���� ���⿼���к��ȵļ��㡢�����Ի�ѧ��Ӧ��Ӱ���Լ���Ӧ���뷴Ӧ����������������Ĺ�ϵ����˹���ɵ�Ӧ�õ�֪ʶ��ͼ���⣬����ʱҪע���ͼ���л�ȡ��ȷ��Ϣ����ȷ�ж�ijһ��Ӧ�����Ȼ��Ƿ��ȣ���˹���ɵ�Ӧ���Ǹ�Ƶ�������������գ���Ŀ�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | �ױ� | B�� | ������ | C�� | 2��2-�������� | D�� | �Զ��ױ� |
| A�� | �Ҵ���ˮ�����������Ƶ���ʯ�ң�Ȼ������ | |
| B�� | �������������ᣩ�����뱥��̼������Һ��Ȼ���Һ | |
| C�� | ���飨��ϩ����ͨ�����Ը��������Һ��ϴ�� | |
| D�� | �屽���壩����������������Һ��Ȼ���Һ |
| A�� | ��ͥ��������������ʢ�Ųˣ�����ʳƷ | |
| B�� | ����[KAl��SO4��2•12H2O]���ᾧˮ���ǻ���� | |
| C�� | Al���������ᷴӦ��������NaOH��Һ��Ӧ | |
| D�� | ���ȷ�Ӧ�Ƿ��ȷ�Ӧ |
| A�� | ijԪ�ػ�̬ԭ�������3��δ�ɶԵ��ӣ����ڲ���2�����ӣ���Ԫ�ط���ΪN | |
| B�� | ijԪ�صĸ�һ�����ӵĵ��Ӳ�ṹ�����ͬ�����̬ԭ�Ӽ۵����Ų�ʽΪ[Ne]3s23p5 | |
| C�� | ijԪ�ص����������ӵ�3d�ܼ�Ϊ����������̬ԭ���Ų�ʽΪ[Ar]3d64s2 | |
| D�� | ijԪ�ػ�̬ԭ�ӵ�M��ȫ������N��û�гɶԵ��ӣ�ֻ��һ��δ�ɶԵ��ӣ����̬ԭ�ӵĵ����Ų�ʽΪ[Ar]3d54s1 |
| A�� | ������̼ԭ�Ӳ���һ��ֱ���� | B�� | ���ղ��ܹ�����ȡ����Ӧ | ||
| C�� | ��ʯ�ͷ����һ�ֲ�Ʒ | D�� | �ȶ������Һ�� |
 ��ѧ����ᾭ�ý��衢��̬�������衢���ཡ�����ϡ�����������������أ�
��ѧ����ᾭ�ý��衢��̬�������衢���ཡ�����ϡ�����������������أ�