��Ŀ����
16�������£������й���Һ���������ʵ���Ũ�ȹ�ϵ����ȷ���ǣ�������| A�� | ��0.1mol•L-1pH=2��HF��Һ��ˮϡ�ͣ�$\frac{c��{H}^{+}��}{c��HF��}$���� | |
| B�� | ��0.2 mol•L-1NaHCO3��Һ�м�������0.1 mol•L-1NaOH��Һ��c��HCO3-����c��CO32-����c��OH-����c��H+�� | |
| C�� | Ũ�Ⱦ�Ϊ0.1 mol•L-1��NH3•H2O��Һ��NH4Cl��Һ�������ϣ�c��NH4+��+c��NH3•H2O��=2c��Cl-�� | |
| D�� | Ϊȷ��ij��H2A��ǿ�ỹ�����ᣬ�ɲ�NaHA��Һ��pH����pH��7����H2A�������pH��7����H2A��ǿ�� |
���� A����������Һ��������Һ���ڵ���ƽ�⣬��ˮϡ�ʹٽ����룬�����ӵ����ʵ��������������ʵ�����С��
B����Ӧ������Ϊ��Ũ�ȵ�̼�����ƺ�̼���ƣ�̼������ӵ�ˮ��̶ȴ���̼��������ӣ���c��HCO3-����c��CO32-����
C�����ݻ��Һ�е������غ��жϣ�
D��NaHA��Һ��pH��7��������HA-�ĵ���̶ȴ�����ˮ��̶ȣ����ܾݴ��ж�H2Aǿ�ᣮ
��� �⣺A����������������ʴ��ڵ���ƽ�⣬��ˮϡ������ٽ����룬���������ʵ��������������ʵ�����С����$\frac{c��{H}^{+}��}{c��HF��}$ʼ�ձ�������A��ȷ��
B����0.2 mol•L-1NaHCO3��Һ�м�������0.1 mol•L-1NaOH��Һ����Ӧ������Ϊ��Ũ�ȵ�̼���ƺ�̼�����ƣ�����̼������ӵ�ˮ��̶ȴ���̼��������ӣ���c��HCO3-����c��CO32-������Һ������Ũ�ȴ�СΪ��c��HCO3-����c��CO32-����c��OH-����c��H+������B��ȷ��
C��Ũ�Ⱦ�Ϊ0.1 mol•L-1��NH3•H2O��Һ��NH4Cl��Һ�������ϣ����������غ�ɵã�c��NH4+��+c��NH3•H2O��=2c��Cl-������C��ȷ��
D��Ϊȷ��ij��H2A��ǿ�ỹ�����ᣬ�ɲ�NaHA��Һ��pH����pH��7����H2A�����������Һ��pH��7������ȷ��H2A��ǿ�ᣬҲ����Ϊ���ᣬ����HA-�ĵ���̶ȴ�����ˮ��̶ȣ���Һ��ʾ���ԣ���D����
��ѡD��
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ��漰������ʵĵ��뼰��Ӱ�졢����ϵĶ����жϡ�����Ũ�ȴ�С�Ƚϵ�֪ʶ����ȷ����غ㡢�����غ㼰�ε�ˮ��ԭ��Ϊ���ؼ�������������ѧ���ķ������������������Ӧ��������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ���ͬλ��������ԭ�ӵķ��ţ�13H | |
| B�� | Mg2+�Ľṹʾ��ͼ�� | |
| C�� | �����ĵ���ʽ�� | |
| D�� | Ca��ClO��2�ĵ��뷽��ʽ��Ca��ClO��2=Ca2++2ClO- |
| A�� | �ܱ������У�9.6 g�����11.2 g���ۻ�ϼ�������17.6 g������ʱ���ų�19.12 kJ��������Fe��s��+S��s���TFeS��s����H=-95.6 kJ•mol-1 | |
| B�� | ϡ������0.1 mol•L-1 NaOH��Һ��Ӧ��H+��aq��+OH-��aq��=H2O��l����H=-57.3 kJ•mol-1 | |
| C�� | ��֪1 mol������ȫȼ������Һ̬ˮ���ų�������Ϊ285.5 kJ����ˮ�ֽ���Ȼ�ѧ����ʽΪ2H2O��l���T2H2��g��+O2��g����H=+285.5 kJ•mol-1 | |
| D�� | ��֪2C��s��+O2��g��=2CO��g����H=-221 kJ•mol-1����C��ȼ���ȡ�H=-110.5 kJ•mol-1 |
| A�� | CH3-CH=CH-CHO | B�� | CH3-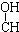 - -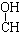 -CH3 -CH3 | ||
| C�� | HOCH2-CH2-CH=CH-CHO | D�� | HOCH2-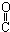 -CH2-CHO -CH2-CHO |
| A�� | Mg��Al��Cu���Էֱ����û�����ֱ�Ӽ��ȷ��͵�ⷨұ���õ� | |
| B�� | С�մ�����������ȸ������ɼ�����������θ������һ��ҩ�� | |
| C�� | S��C��Si������Ȼ���к����ḻ�ķǽ���Ԫ�أ�����Ԫ�صĶ������ﶼ�������������������Ӧ���������ᷴӦ | |
| D�� | ���������ﶼ���ɽ���Ԫ�غ���Ԫ����ɣ���K2O��CuO��Na2O��Na2O2��Mn2O7��Fe2O3��ȫ���ɽ���Ԫ�غ���Ԫ����ɵģ���˶�Ϊ���������� |
| A�� | �ڱ���£�22.4L SO3��22.4L C2H4ԭ�Ӹ�����Ϊ2��3 | |
| B�� | ��78g Na2O2�����CO2��Ӧ��CO2ת�Ƶĵ�����Ϊ2NA | |
| C�� | ��0.2mol H2SO4��Ũ����������ͭ��ַ�Ӧ������SO2���ӵ���Ŀ����0.1NA | |
| D�� | ��⾫��ͭʱ����������������32g��������ת�Ƶĵ��ӵ���ĿΪNA |
| A�� | ˮ | B�� | ����������ͭ��Һ | ||
| C�� | ̼������Һ | D�� | ��ɫʯ����Һ |
 ��
��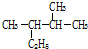 ��ϵͳ����������Ϊ2��3-�������飻
��ϵͳ����������Ϊ2��3-�������飻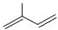 ��
��