��Ŀ����
16�� ijѧ������ͼ��ʾװ��֤����ͱ��ķ�Ӧ��ȡ����Ӧ�����Ǽӳɷ�Ӧ����Ҫʵ�鲽�����£�
ijѧ������ͼ��ʾװ��֤����ͱ��ķ�Ӧ��ȡ����Ӧ�����Ǽӳɷ�Ӧ����Ҫʵ�鲽�����£��ټ�������ԣ�Ȼ������ƿ�м���һ�����ı���Һ�壮
������ƿ�м���ij��Һ������С�Թ��м���CCl4�������ұߵij����ܿڽ���CCl4Һ���£�
�۽�װ��A�еĴ���˿С�����²�����Һ�У�
����д���пհף�
��1��С�Թ����Һ���ǣ������ƣ�ˮ��������������HBr���壬װ��B����ƿ��С�Թ���CCl4�����������ջӷ�����������
��2����Ӧ������ƿ�еμӣ������ƣ���������Һ���������е���ɫ�������ɣ��������Ǽ��������ӣ�װ��B�����������Ƿ�������
���� ��ͼ��֪��A�з���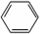 +Br2$\stackrel{Fe}{��}$
+Br2$\stackrel{Fe}{��}$ +HBr��װ��B����ƿ��С�Թ���CCl4�����塢�屽��С�Թ����Һ��Ϊˮ������HBr���壬Ȼ��μ����������������ӣ��Դ������
+HBr��װ��B����ƿ��С�Թ���CCl4�����塢�屽��С�Թ����Һ��Ϊˮ������HBr���壬Ȼ��μ����������������ӣ��Դ������
��� �⣺�������ӷ�Ӧ�����ɸ����������ȡ����Ӧ����HBr��
��1��С�Թ����Һ����ˮ��������������HBr���壬װ��B����ƿ��С�Թ���CCl4�����������ջӷ�����������
�ʴ�Ϊ����ˮ������HBr���壻���ջӷ�����������
��2����Ӧ������ƿ�еμ���������Һ��HBr����������Ӧ����AgBr�������������е���ɫ�������ɣ��������Ǽ��������ӣ���֪����ȡ����Ӧ��HBr��������ˮ��ˮ�����岻��ֱ�ӽӴ�����װ��B�����������Ƿ��������ʴ�Ϊ�����������е���ɫ�������ɣ����������ӣ���������
���� ���⿼��ʵ��װ���ۺϼ��л�����Ʊ�ʵ�飬Ϊ��Ƶ���㣬���շ�Ӧԭ����ʵ��װ�õ�����Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
6���й��������ʼ���;��˵������ȷ���ǣ�������
| A�� | �����������ڹ��յ���������ȡ������һ�ȼ��� | |
| B�� | �����������У��پ���ϩ���ھ۱�ϩ���۾�����ϩ���ܾ۱���ϩ��������ڲ�������˫�� | |
| C�� | ĵ��������һ��������ֲ����֬������ʹ���Ը��������Һ��ɫ | |
| D�� | ����������ϩ�;���ϩ���ȼ������������������� |
7������˵��������ǣ�������
| A�� | ���뵼����ϵ�Ԫ�ش����λ�����ڱ��н���Ԫ�غͷǽ���Ԫ�صĽ��紦 | |
| B�� | ũҩ�г����е�Ԫ��ͨ����Ԫ�����ڱ������Ϸ������� | |
| C�� | ���ɴ�����Ԫ��ͨ����Ԫ�����ڱ������·������� | |
| D�� | �����ڱ�����Ԫ����Ѱ�������º���ʴ�ĺϽ���ϵ�Ԫ�� |
4������������ȷ���ǣ�������
| A�� | ���ᡢ�Ȼ�����Ϊ���� | B�� | ���ᡢ���Ǿ����ڵ���� | ||
| C�� | ˮ��������ˮ��Ϊ����� | D�� | �����ơ���������Ϊ���������� |
11����NAΪ�����ӵ�����������������ȷ���ǣ�������
| A�� | ��״���£�11.2LNO��11.2LO2��Ϻ�����������Ϊ0.75NA | |
| B�� | 0.1molNa2O2�������0.3NA������ | |
| C�� | pH=13��1.0LBa��OH��2��Һ�к��е�OH-��ĿΪ0.2NA | |
| D�� | ��״���£�22.4L�״��к��е���ԭ����Ϊ1.0NA |
1����״�������ֵ����ʵ����Ŀ�ȼ���干1.68L������һ����������������������ȫȼ�գ���������ͨ����������ʯ��ˮ���õ��İ�ɫ��������Ϊ15.0g������������ʯ������ȼ�ղ������9.3g���������ֻ���������Ϊ��������
| A�� | H2��C2H4 | B�� | CO��C2H4 | C�� | H2��C4H6 | D�� | CO ��C3H6 |
8����F-������ͬ���������͵����������ǣ�������
| A�� | Cl- | B�� | OH- | C�� | NH${\;}_{4}^{+}$ | D�� | NH3 |
6�������йص������Һ������Ũ�ȹ�ϵ����������ȷ���ǣ�������
| A�� | 0.1mol•L-1�ģ�NH4��2Fe��SO4��2��Һ�У�c��Fe2+��+c��NH4+��+c��H+��=c��SO42-��+c��OH-�� | |
| B�� | ����������ʵ���Ũ�ȵ�Na2CO3��NaHCO3��Һ��ϣ�2c��CO32-��+2c��HCO3-��+2c��H2CO3��=3c��Na+�� | |
| C�� | 0.2mol•L-1��CH3COOH��Һ��0.1mol•L-1��NaOH��Һ�������ϣ�c��CH3COOH��+2c��H+��=c��CH3COO-��+2c��OH-�� | |
| D�� | PH��ͬ��CH3COONa��Һ��NaHCO3��Һ��NaOH��Һ��c��NaHCO3����c��CH3COONa����c��NaOH�� |
 ��ͼ��ʾ��ͼ�в�������δ��������һ�ݻ�Ϊ300mL��ע��������������ƿ��500mL�������ӣ���ƿ����0.384g CuƬ��������ƿ�м���18mL 2.5mol•L-1��ϡHNO3��Һ����������������ס��Ƥ����סƿ�ڣ������������Ϊ��״���µģ��Իش�
��ͼ��ʾ��ͼ�в�������δ��������һ�ݻ�Ϊ300mL��ע��������������ƿ��500mL�������ӣ���ƿ����0.384g CuƬ��������ƿ�м���18mL 2.5mol•L-1��ϡHNO3��Һ����������������ס��Ƥ����סƿ�ڣ������������Ϊ��״���µģ��Իش�