��Ŀ����
һλѧ����������������Ӧ��IJ������̽����
(1)�������
����1������ΪFeO��
����2�� ��
(2)��������
��ѧ��ͨ���������ϵ�֪�������������������У��������������ȶ�������������ȶ��������¼��ױ�����������������(��ɫ�ɺ�ɫ��ɺ�ɫ)��
ͨ���������Ͽ��Եó��ij�������Ϊ ��
(3)����ʵ��
��ѧ�����������װ�ý���������������Ӧ��ʵ�顣�����������ʵ�鲽�貹��������
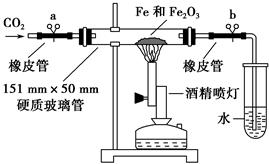
�ٰ���ͼװ�����Ӻ�����(�ݲ�װ��ҩƷ)�� ��
�ڳ�ȡ1 g��ԭ�����ۺ�5 g��������ĩ����Ͼ��Ⱥ�ƽ̯�ڲ������в���
���ɿ��������ɼУ� �����ɼ��ϵ��ɼ�a������ʼ����ҩƷ��
�ܴ�Լ4�������ң���ɫ��ĩȫ����ڣ��ټ��ϵ��ɼ�b��Ȼ��ֹͣ���ȣ��ȵ���������ȴ�����£�������ɫ��ĩ��
(4)���ṩ����ҩƷ����֤ʵ��õ��ĺ�ɫ��ĩ�ijɷ֡�������ϡ���ᡢKSCN��Һ������KMnO4��Һ���Թܡ���ͷ�ιܡ�
(5)ʵ����ۣ�������������Ӧ�Ļ�ѧ����ʽΪ ��
(1)�������
����1������ΪFeO��
����2�� ��
(2)��������
��ѧ��ͨ���������ϵ�֪�������������������У��������������ȶ�������������ȶ��������¼��ױ�����������������(��ɫ�ɺ�ɫ��ɺ�ɫ)��
ͨ���������Ͽ��Եó��ij�������Ϊ ��
(3)����ʵ��
��ѧ�����������װ�ý���������������Ӧ��ʵ�顣�����������ʵ�鲽�貹��������

�ٰ���ͼװ�����Ӻ�����(�ݲ�װ��ҩƷ)�� ��
�ڳ�ȡ1 g��ԭ�����ۺ�5 g��������ĩ����Ͼ��Ⱥ�ƽ̯�ڲ������в���
���ɿ��������ɼУ� �����ɼ��ϵ��ɼ�a������ʼ����ҩƷ��
�ܴ�Լ4�������ң���ɫ��ĩȫ����ڣ��ټ��ϵ��ɼ�b��Ȼ��ֹͣ���ȣ��ȵ���������ȴ�����£�������ɫ��ĩ��
(4)���ṩ����ҩƷ����֤ʵ��õ��ĺ�ɫ��ĩ�ijɷ֡�������ϡ���ᡢKSCN��Һ������KMnO4��Һ���Թܡ���ͷ�ιܡ�
| ʵ�鲽�� | Ԥ������ͽ��� |
| | |
(5)ʵ����ۣ�������������Ӧ�Ļ�ѧ����ʽΪ ��
(1)����ΪFe3O4
(2)Fe��Fe2O3��Ӧ�IJ���ΪFe3O4(������������)
(3)�ټ��ϵ��ɼ�a���ɿ����ɼ�b���Ȳ����ܣ������ܿ�������ð����ֹͣ����һ��ʱ��������γ�һ��ˮ������װ�ò�©�� ��ͨ�봿������Ķ�����̼���壬����������Ŀ�����������
(4)
(5)Fe��4Fe2O3 3Fe3O4
3Fe3O4
(2)Fe��Fe2O3��Ӧ�IJ���ΪFe3O4(������������)
(3)�ټ��ϵ��ɼ�a���ɿ����ɼ�b���Ȳ����ܣ������ܿ�������ð����ֹͣ����һ��ʱ��������γ�һ��ˮ������װ�ò�©�� ��ͨ�봿������Ķ�����̼���壬����������Ŀ�����������
(4)
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1���ô���������ɫ��ĩ | ��ɫ��ĩ�ڿ����в���ɫ�����ܱ�����ȫ��������˵����ɫ��ĩ��û��FeO |
| ����2��ȡ������ɫ��ĩ�������Թ��У�����ϡ���ᣬ��ʹ��ĩ�ܽ⣬Ȼ����뼸��KSCN��Һ | ������ð������Һ���ɫ��˵�����۹�����������ΪFe3O4 |
 3Fe3O4
3Fe3O4ʵ��Ҫ�����������н��С�����Fe3O4ʱ��һ��Ҫ�������Ĵ����ų�Fe2O3��FeO�ĸ��ţ�����Ҫ���飫3�������ӵĴ��ڣ��ų����ĸ��š�

��ϰ��ϵ�д�
�����Ŀ
 ԭ�������������������ȼ��̶�װ��ʡ�ԣ��Ʊ����������CO������CO��ԭCuO��ĩ��
ԭ�������������������ȼ��̶�װ��ʡ�ԣ��Ʊ����������CO������CO��ԭCuO��ĩ��
 2 Cu2O + O2������ Cu2O +2H+
2 Cu2O + O2������ Cu2O +2H+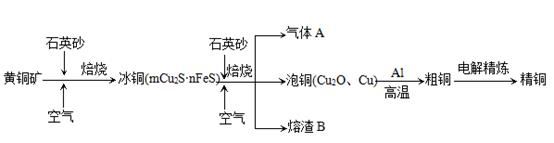
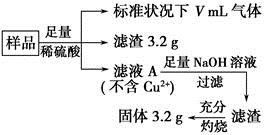

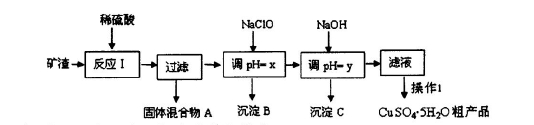
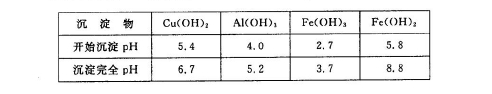

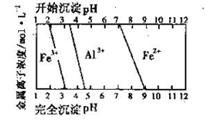

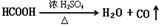
 FeCO3(s)��H2CO3(aq)��ƽ�ⳣ��Ϊ_______��
FeCO3(s)��H2CO3(aq)��ƽ�ⳣ��Ϊ_______��