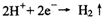��Ŀ����
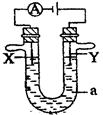
��8�֣���̼�����缫����Na+��Cu2+��Cl����SO42����������ѡ���ʵ�������ɵ���ʣ��������Һ��д������������һ�ֵ���ʵĻ�ѧʽ��
�ŵ������ų�H2�������ų�O2ʱ��������� ��
�Ƶ��������������������ų�O2ʱ��������� ��
�ǵ������ų�H2�������ų�Cl2ʱ��������� ��
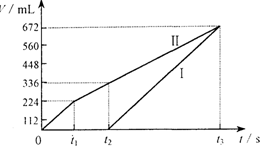
(4)ͨ�����ӵ����ʵ��������������Ľ��������ʵ�����������������������ʵ���֮��Ϊ4��2��1ʱ������ʵĻ�ѧʽ�� ��
�ŵ������ų�H2�������ų�O2ʱ��������� ��
�Ƶ��������������������ų�O2ʱ��������� ��
�ǵ������ų�H2�������ų�Cl2ʱ��������� ��
(4)ͨ�����ӵ����ʵ��������������Ľ��������ʵ�����������������������ʵ���֮��Ϊ4��2��1ʱ������ʵĻ�ѧʽ�� ��
��1��Na2SO4 (2) CuSO4 (3) NaCl (4)CuSO4
��1�������ų�H2�������ų�O2��˵���൱�ڵ��ˮ���������ʿ�����Na2SO4��
��2����ˮ��Һ��Na+�����ܷŵ磬����ֻ����Cu2+�ŵ硣�����ų�O2��˵����OH���ŵ磬���Բ��ܻ�ԭ�����ӣ�������CuSO4��
��3�������ų�H2��˵������Һ�е������ӷŵ磬��˲��ܺ���ͭ���ӡ������ų�Cl2��˵����Һ�б��뺬�������ӣ���������ΪNaCl��
��4����������������˵�����뺬��ͭ���ӡ���������������ͭ�����ʵ�����������������������ʵ���֮��Ϊ2��1����ÿ����1mol�����壬��Ӧ�о�ת��4mol���ӣ�����������������������ΪCuSO4��
��2����ˮ��Һ��Na+�����ܷŵ磬����ֻ����Cu2+�ŵ硣�����ų�O2��˵����OH���ŵ磬���Բ��ܻ�ԭ�����ӣ�������CuSO4��
��3�������ų�H2��˵������Һ�е������ӷŵ磬��˲��ܺ���ͭ���ӡ������ų�Cl2��˵����Һ�б��뺬�������ӣ���������ΪNaCl��
��4����������������˵�����뺬��ͭ���ӡ���������������ͭ�����ʵ�����������������������ʵ���֮��Ϊ2��1����ÿ����1mol�����壬��Ӧ�о�ת��4mol���ӣ�����������������������ΪCuSO4��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ