��Ŀ����
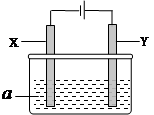
��6�֣����ԭ���ڻ�ѧ��ҵ�����Ź㷺��Ӧ�á�ͼ�е���aΪ���Һ��X��Y������缫�塣��
(1)��X��Y��Ϊ���Ե缫��aΪ���͵�NaCI��Һ������ʱ����Y�缫��Ӧ����ķ���������������������������
(2)��X��Y�ֱ�Ϊʯī������A��Ϊ���͵�NaCl��Һ��������������ɵİ�ɫ��������¶���ڿ����У��ɹ۲쵽������Ϊ����������������������������
(3)��X��Y��Ϊ���Ե缫��aΪһ��Ũ�ȵ�����ͭ��Һ��ͨ��һ��ʱ�����������Һ�м���0��1 mol Cu(OH)2��ǡ�ûָ����ǰ��Ũ�Ⱥ�pH�����������ת�Ƶĵ��ӵ����ʵ���Ϊ����������

(1)��X��Y��Ϊ���Ե缫��aΪ���͵�NaCI��Һ������ʱ����Y�缫��Ӧ����ķ���������������������������
(2)��X��Y�ֱ�Ϊʯī������A��Ϊ���͵�NaCl��Һ��������������ɵİ�ɫ��������¶���ڿ����У��ɹ۲쵽������Ϊ����������������������������
(3)��X��Y��Ϊ���Ե缫��aΪһ��Ũ�ȵ�����ͭ��Һ��ͨ��һ��ʱ�����������Һ�м���0��1 mol Cu(OH)2��ǡ�ûָ����ǰ��Ũ�Ⱥ�pH�����������ת�Ƶĵ��ӵ����ʵ���Ϊ����������
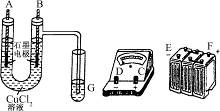
(1)��ʪ��ĵ��۵⻯����ֽ����Y��֧�ܿڣ���ֽ������˵������������
(2)��ɫ����Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ (3)0��4 mol
(2)��ɫ����Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ (3)0��4 mol
��1��Y�������������Y�������������������������������ԣ�����ʪ��ĵ��۵⻯����ֽ����Y��֧�ܿڣ���ֽ������˵�����������ɡ�
��2����ʱ����������������ʧȥ���ӣ������������ӡ����������ӷŵ磬�Ӷ������������ƣ��������������������������������������ȶ������ױ���������������������������������ǰ�ɫ����Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ��
��3����������Һ�м���0��1 mol Cu(OH)2��ǡ�ûָ����ǰ��Ũ�Ⱥ�pH��˵����Ӧ��������ͭ��������������������������ԭ���غ��֪��������0.1mol������ת�Ƶ�����0.1mol��4��0.4mol��
��2����ʱ����������������ʧȥ���ӣ������������ӡ����������ӷŵ磬�Ӷ������������ƣ��������������������������������������ȶ������ױ���������������������������������ǰ�ɫ����Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ��
��3����������Һ�м���0��1 mol Cu(OH)2��ǡ�ûָ����ǰ��Ũ�Ⱥ�pH��˵����Ӧ��������ͭ��������������������������ԭ���غ��֪��������0.1mol������ת�Ƶ�����0.1mol��4��0.4mol��

��ϰ��ϵ�д�
 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
�����Ŀ