��Ŀ����
9��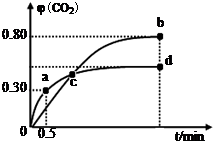 ����I2O5������CO��Ⱦ�����ⶨCO����ӦΪ��
����I2O5������CO��Ⱦ�����ⶨCO����ӦΪ��5CO��g��+I2O5��s��?5CO2��g��+I2��s������H1
��1����֪��2CO��g��+O2��g��?2CO2��g������H2
2I2��s��+5O2��g��?2I2O5��s������H3
���H1=2.5��H2-0.5��H3���ú���H2�͡�H3�Ĵ���ʽ��ʾ����
��2����ͬ�¶��£���װ������I2O5�����2L�����ܱ�������ͨ��2molCO�����CO2����������գ�CO2����ʱ��t�仯������ͼ����ش�
�ٴӷ�Ӧ��ʼ��a��ʱ�ķ�Ӧ����Ϊv��CO��=0.6mol•L-1•min-1��b��ʱ��ѧƽ�ⳣ������ʽ�����㣩Kb=1024��
��d��ʱ���¶Ȳ��䣬�����������ѹ����ԭ����һ�룬����ͼ�в��仭��CO2��������ı仯���ߣ�
������˵������ȷ����C��������ĸ��ţ�
A�������������ܶȲ��䣬������Ӧ�ﵽƽ��״̬
B�������¶��£�c��ʱ��ϵ�л�������ƽ����Է����������
C������I2O5��Ͷ�������������CO��ת����
D��b���d��Ļ�ѧƽ�ⳣ����Kb��Kd
��3����250mL����״��������CO��ij������Ʒͨ��ʢ������I2O5�ĸ���ܣ�170���³�ַ�Ӧ����ˮ-�Ҵ�Һ����ܽ����I2�����ݵ�100mL��ȡ25.00mL����0.0100mol•L-1 Na2S2O3����Һ�ζ�����ҺӦװ�ڼ�ʽ�ζ��ܣ����ʽ�ζ��ܡ���ʽ�ζ��ܡ�����ѡ���ָʾ��Ϊ������Һ�������ı���Һ20.00mL������Ʒ����CO���������Ϊ17.92%������֪��������Ʒ�������ɷ���I2O5����Ӧ��2Na2S2O3+I2=2NaI+Na2S4O6��
���� ��1�����ݸ�˹���ɺ���֪��Ӧ�ɵã�
��2������ʼ����a��ʱCO2�����������������������������ʽ���㼴������ӷ�Ӧ��ʼ��a��ʱ�ķ�Ӧ����Ϊv��CO��������b��ʱCO2����������գ�CO2���ƽ��Ũ��[CO]��[CO2]�������b��ʱ��ѧƽ�ⳣ��Kb��
�ڶ��ڷ�Ӧǰ����������ʵ�������ķ�Ӧ����ѹƽ�ⲻ�ƶ���
��A����Ϊ����Ϊ���ݣ�����Ӧǰ�����������仯�����������������ܶ��DZ�����������ʱ������Ӧ�ﵽƽ��״̬��
B��c��Ϊ���㣬���������ʵ����ֱ���ȣ�
C��I2O5Ϊ���壬��������Ͷ������ƽ����Ӱ�죻
D��b���d��ʱ������CO2���������˵�����еij̶ȴ���ѧƽ�ⳣ����Kb��Kd��
��3��Na2S2O3����Һ��Ϊˮ��ʼ��ԣ�����Ҫ���ڼ�ʽ�ζ����У�����������Һ����ɫ�����Կ����õ�����Һ��ָʾ�������ݷ���ʽ��5CO��g��+I2O5��s��?5CO2��g��+I2��s����2Na2S2O3+I2=2NaI+Na2S4O6�й�ϵʽ�ã�5CO����I2������2Na2S2O3��Ȼ����㼴�ɣ�
��� �⣺��1�����ݸ�˹���ɺ���֪��Ӧ��2CO��g��+O2��g��?2CO2��g����H 2-----��
2I2��s��+5O2��g��?2I2O5��s����H 3-----��
��֪��Ӧ��5CO��g��+I2O5��s��?5CO2��g��+I2��s�����ɢ١�2.5-�ڡ�0.5�õ������H 1=2.5��H2-0.5��H3��
�ʴ�Ϊ��2.5��H2-0.5��H3��
��2����a��ʱ��5CO��g��+I2O5��s��?5CO2��g��+I2��s��
��ʼ��/mol 2 0
ת����/mol x x
a����/mol 2-x x
����a��ʱCO2����������գ�CO2��=$\frac{x}{2}$=0.30����x=0.6mol
��ӷ�Ӧ��ʼ��a��ʱ�ķ�Ӧ����Ϊv��CO��=$\frac{0.6mol}{2L��0.5min}$=0.6mol•L-1•min-1��
b��ʱ��5CO��g��+I2O5��s��?5CO2��g��+I2��s��
��ʼ��/mol 2 0
ת����/mol y y
b����/mol 2-y y
����b��ʱCO2����������գ�CO2��=$\frac{y}{2}$=0.80����y=1.6mol��[CO]=0.2mol•L-1��[CO2]=0.8mol•L-1
b��ʱ��ѧƽ�ⳣ��Kb=$\frac{[CO{\;}_{2}]{\;}^{5}}{[CO]{\;}^{5}}$=1024��
�ʴ�Ϊ��0.6mol•L-1•min-1��1024��
��d��ʱ���¶Ȳ��䣬�����������ѹ����ԭ����һ�룬����ѹ�������ڷ�Ӧǰ����������ʵ������䣬����ƽ�ⲻ�ƶ���CO2����������䣬
�ʴ�Ϊ��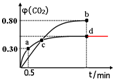 ��
��
��A����Ϊ����Ϊ���ݣ�����Ӧǰ�����������仯�����������������ܶȲ���ʱ��������Ӧ�ﵽƽ��״̬����A��ȷ��
B��c��Ϊ���㣬�������ʵ����ֱ���ȣ����������¶��£���ϵ�л�������ƽ����Է���������ȣ���B��ȷ��
C��I2O5Ϊ���壬��������Ͷ������ƽ����Ӱ�죬CO��ת���ʲ��䣬��C����
D��b���d��ʱ������CO2���������˵�����еij̶ȴ���ѧƽ�ⳣ����Kb��Kd����D��ȷ��
�ʴ�Ϊ��C��
��3��Na2S2O3����Һ��Ϊˮ��ʼ��ԣ�����Ҫ���ڼ�ʽ�ζ����У�����������Һ����ɫ�����Կ����õ�����Һ��ָʾ�������ݷ���ʽ��5CO��g��+I2O5��s��?5CO2��g��+I2��s����2Na2S2O3+I2=2NaI+Na2S4O6�й�ϵʽ�ã�
5CO����I2������2Na2S2O3
5 2
n��CO�� 0.01��0.02��$\frac{100}{25}$mol
�� n��CO��=0.002mol V��CO��=0.0448L=44.8mL
����Ʒ����CO���������Ϊ��$\frac{44.8}{250}$��100%=17.92%��
�ʴ�Ϊ����ʽ�ζ��ܣ�������Һ��17.92%��
���� ���⿼���˸��ݸ�˹�������ʱ䡢���ʡ�ƽ�⡢������ԭ��Ӧ�����⣬�ۺ��Խ�ǿ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | 44g CO2������ԭ����ΪNA | |
| B�� | ����������ˮ��Ӧʱ������0.1mol����ת�Ƶĵ�����Ϊ0.2NA | |
| C�� | 23gNa��������O2�г��ȼ��ʧȥ2mole-����Na2O2 | |
| D�� | ��״���£�5.6LCO2������Na2O2��Ӧת�Ƶĵ�����Ϊ0.5 NA |
| A�� | �մ� | B�� | ˮ�� | C�� | ���� | D�� | ���� |
T1�¶��µIJ���ʵ������Ϊ
| t/s | 0 | 500 | 1000 | 1500 |
| c��N2O5��mol•L-1 | 5.00 | 3.52 | 2.50 | 2.50 |
| A�� | �����������䣬T2�¶��·�Ӧ��1000sʱN2O5��g��Ũ��Ϊ2.98mol•L-1����T1��T2 | |
| B�� | T1�¶��µ�ƽ�ⳣ��ֵΪK1=125��1000sʱת����Ϊ50% | |
| C�� | 500s��N2O5�ֽ�����Ϊ2.96��10-3 mol•L-1•s-1 | |
| D�� | �÷�Ӧ��S��0����Ȼ�����ȷ�Ӧ���ڳ�����Ҳ���Է����� |
| A�� | ��֪H+��aq��+OH-��aq���TH2O��l����H=-57.3 kJ•mol-1����H2SO4��Ba��OH��2�ķ�Ӧ�ȡ�H=2����-57.3��kJ•mol-1 | |
| B�� | ȼ�ϵ���н��״�����ת��Ϊ�������Ȼ�ѧ����ʽ��CH3OH��g��+$\frac{1}{2}$O2��g���TCO2��g��+2H2��g�� ��H=-192.9 kJ•mol-1����CH3OH��g����ȼ����Ϊ192.9 kJ•mol-1 | |
| C�� | H2��g����ȼ������285.8 kJ•mol-1����2H2O��g���T2H2��g��+O2��g����H=+571.6 kJ•mol-1 | |
| D�� | ������ȼ������2800 kJ•mol-1����$\frac{1}{2}$C6H12O6��s��+3O2��g��=3CO2��g��+3H2O��l����H=-1400 kJ•mol-1 |
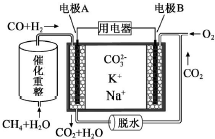 һ������̼����ȼ�ϵ��ԭ��ʾ����ͼ�������йظõ�ص�˵����ȷ���ǣ�������
һ������̼����ȼ�ϵ��ԭ��ʾ����ͼ�������йظõ�ص�˵����ȷ���ǣ�������| A�� | ���ô��ֵ�ص��ͭ������ͭ����������19.2g�����������ı�״����2.24L CH4 | |
| B�� | �缫A��H2����ĵ缫��ӦΪ��H2+2OH--2e-=2H2O | |
| C�� | ��ع���ʱ��CO32-��缫B�ƶ� | |
| D�� | �缫B�Ϸ����ĵ缫��ӦΪ��O2+2CO2+4e-=2CO32- |
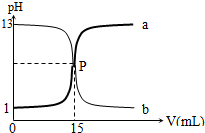 ��ͼ������������������Һ�ĵζ�����a��b��
��ͼ������������������Һ�ĵζ�����a��b��