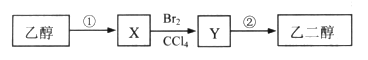
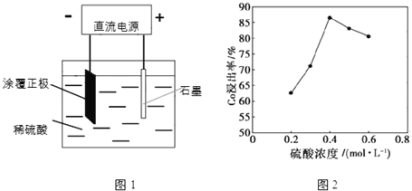
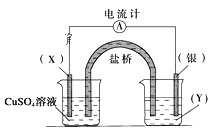
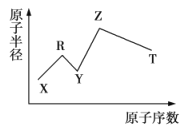
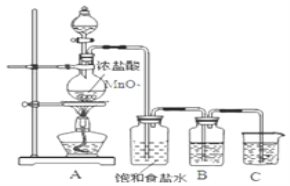
��Ŀ����
����Ŀ����A��I��Ԫ����ѡ����������Ԫ�أ���Ҫ��ش��������⣺
������ | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
1 | A | |||||||
2 | D | E | G | |||||
3 | B | C | J | F | H | I |
(1)ֻ�и��۶������۵���______(��Ԫ������)��
(2)����������ˮ����������ǿ����____(�ѧʽ)��
(3)A�ֱ���D��E��F��G��H�γɵĻ������У����ȶ��Ļ�����Ľṹʽ _____��
(4)��B��C��D��J�� E��F��G��H�У�ԭ�Ӱ뾶������____(��ԭ�ӷ���)��
(5)��������Ԫ���н�������ǿ��Ԫ�ص�ԭ�ӽṹʾ��ͼ______��
(6)C����������������Һ��Ӧ�����ӷ���ʽ________________________��
(7)�õ���ʽ��ʾE���ʵ��γɹ���_______________________
(8)д��ұ��B���ʵĻ�ѧ����ʽ____________________��
(9)D������⻯���ȼ����Ϊ890.3 kJ/mol��д��D������⻯��ȼ���ȵ��Ȼ�ѧ����ʽ_____��
(10)д��NaHSO4����ʱ���뷽��ʽ______________________��
���𰸡��� HClO4 H��F Na ![]() 2Al+2OH-+2H2O=2
2Al+2OH-+2H2O=2![]() +3H2��
+3H2�� ![]() 2NaCl(����)
2NaCl(����)![]() 2Na+Cl2�� CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H =-890.3kJ/mol NaHSO4(����)=Na++
2Na+Cl2�� CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H =-890.3kJ/mol NaHSO4(����)=Na++![]()
��������
���ݸ�Ԫ����Ԫ�����ڱ���λ�ÿ�֪A~J�ֱ�ΪH��Na��Al��C��N��P��F��Cl��Ar��Si��
(1)��Ԫ��Ϊ�ǽ�������ǿ��Ԫ�أ�ֻ������û�и��ۣ�
(2)�ǽ�����Խǿ����������ˮ���������Խǿ��Ԫ�����ڱ���Խ�����Ͻǵ�����Ԫ�أ��ǽ�����Խǿ����Fû��������ۣ���������������ˮ����������ǿ����ClԪ�أ���Ӧ����ΪHClO4��
(3)AΪHԪ�أ��ǽ�����Խǿ���⻯��Խ�ȶ���D��F��F��G��H�зǽ�������ǿ����G����Ӧ��Ԫ�أ��⻯��ĽṹʽΪH��F��
(4)ͬ��������Ԫ����������ԭ�Ӱ뾶���μ�С��ͬ�������϶���ԭ�Ӱ뾶������������NaԪ�ص�ԭ�Ӱ뾶���
(5)������ǿ��Ԫ��ΪNaԪ�أ���ԭ�ӽṹʾ��ͼΪ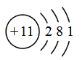 ��
��
(6)CΪAl��Al��NaOH��Һ��Ӧ����������ƫ�����ƣ����ӷ���ʽΪ2Al+2OH��+2H2O=2![]() +3H2����
+3H2����
(7)EΪNԪ�أ�N2������Nԭ��ͨ�����ۼ����ɣ����γɹ��̿��Ա�ʾΪ![]() ��
��
(8)BΪNa��Ϊ���ý�����ͨ���������״̬��NaCl��ȡNa���ʣ���ѧ����ʽΪ2NaCl(����)![]() 2Na+Cl2��
2Na+Cl2��
(9)D�ļ��⻯��ΪCH4����ȼ����Ϊ890.3 kJ/mol����1molCH4��ȫȼ������Һ̬ˮ�Ͷ�����̼�ų�890.3kJ��������Ӧ���Ȼ�ѧ����ʽΪ��CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H =-890.3kJ/mol��
(10)����״̬��NaHSO4�ĵ��뷽��ʽΪNaHSO4(����)=Na++![]() ��
��

 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д� ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�