��Ŀ����
11��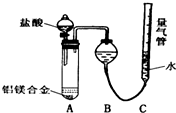 ��þ�Ͻ��ѳ�Ϊ�ִ����졢������������ҵ����Ҫ���ϣ��о���ѧϰС�����λͬѧ��Ϊ�ⶨ��֪��������þ�Ͻ��費������Ԫ�أ���þ����������������������ֲ�ͬʵ�鷽���������Լ���Ϊ����������̽������д���пհף�
��þ�Ͻ��ѳ�Ϊ�ִ����졢������������ҵ����Ҫ���ϣ��о���ѧϰС�����λͬѧ��Ϊ�ⶨ��֪��������þ�Ͻ��費������Ԫ�أ���þ����������������������ֲ�ͬʵ�鷽���������Լ���Ϊ����������̽������д���пհף���̽��һ��
ʵ�鷽������þ�Ͻ�$\stackrel{����}{��}$�ⶨ������������
ʵ��װ�ã��������ۣ�
��1��ijͬѧ�����ʵ��װ�ò������ƣ�Ӧ��A��B֮������һ��װ�м�ʯ�ҵĸ���װ�ã��������ǣ�����Ҫ �����Ҫ������Ҫ������
��2��Ϊȷ�ⶨ��������������ʵ����Ӧע��������ǣ�ֻҪ��д������һ�㣩�����װ�õ������Ի�Ͻ���ȫ�ܽ⣨������������ᣬ�����������C�ĸ߶ȣ�ʹC��Һ����BҺ����ƽ������ȴ�������ٶ�����ȣ�
��̽������
ʵ�鷽������þ�Ͻ�$\stackrel{����������Һ}{��}$���ˣ��ⶨʣ���������
�������ۣ�
��1����ȡһ����������þ�Ͻ��ĩ��Ʒ�����������NaOH��Һ����ַ�Ӧ��ʵ���з�����Ӧ�Ļ�ѧ����ʽ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��2�����ˡ�ϴ�ӡ��������ʣ����壮��δϴ�ӹ��壬���þ������������ƫ�ߣ��ƫ�ߡ���ƫ�͡�����
��̽������
ʵ�鷽������þ�Ͻ� $\stackrel{����������Һ}{��}$���˺����Һ $\stackrel{����}{��}$���ˣ��ⶨ��������
�������ۣ�
��1����ͬѧ��Ϊ�÷��������У���˵�����ɣ�Al��OH��3��������������������������������ɣ�����������������ƣ���
��2����ͬѧ��ΪֻҪ���������Լ���˳�Ϳ��Դﵽʵ��Ŀ�ģ�������Ŀ�����ȷ��
�����õij�����Mg��OH��2��
��3����ͬѧ��ΪҲ����ͨ��������A���ʴ����������ﵽʵ��Ŀ�ģ���A���ʿ�����CO2����д����Ӧ��Ӧ�Ļ�ѧ����ʽ��NaAlO2+H2O+CO2=Al��OH��3��+NaHCO3��
���� ̽��һ����1���Ȼ��⼫������ˮ���ӷ����Ȼ��ⲻӰ����������IJⶨ��������Բ���Ҫ�ӳ���װ�ã�
��2��װ�õ������ԡ��Ͻ��Ƿ���ȫ�ܽ⣨������������ᣬ�����������C�ĸ߶ȣ�ʹC��Һ����BҺ����ƽ������ȴ�������ٶ�������Ȼ�Ӱ��ⶨ�����
̽��������1����������������Һ��Ӧ����ƫ��������������
��2��þ�ϻḽ��ƫ�����Ƶ����ʣ�δϴ�ӵ��²ⶨ��þ������ƫ��
̽��������1��þ���Ͻ���������������Һ�����˵õ�����ƫ��������Һ������������������ƣ��ⶨ�����ȷ��
��2���ȼ������ܽ������Ȼ������Ȼ�þ��Һ����������������Һ�õ�������þ������
��3���������������ɵ���ҺΪƫ��������Һ�����ͨ�����������̼����������������������̼�����ƣ�
��� �⣺̽��һ����1�������Ȼ��⼫������ˮ���ӷ����Ȼ��ⲻӰ����������IJⶨ��������Բ���Ҫ�ӳ���װ�ã�
�ʴ�Ϊ������Ҫ��
��2����Ӧ��װ�õ������ԡ��Ͻ��Ƿ���ȫ�ܽⶼ��Ӱ��ⶨ�����������Ҫ���װ�õ������Ի�Ͻ���ȫ�ܽ⣨������������ᣬ�����������C�ĸ߶ȣ�ʹC��Һ����BҺ����ƽ������ȴ�������ٶ�����ȣ�
�ʴ�Ϊ�����װ�õ������Ի�Ͻ���ȫ�ܽ⣨������������ᣬ�����������C�ĸ߶ȣ�ʹC��Һ����BҺ����ƽ������ȴ�������ٶ�����ȣ���
̽��������1����������������Һ��Ӧ����ƫ����������������Ӧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��2��þ�ϻḽ��ƫ�����Ƶ����ʣ�δϴ�ӵ��²ⶨ��þ������ƫ��þ����������ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
[̽����]��1��þ���Ͻ���������������Һ�����˵õ�����ƫ��������Һ������������������ƣ�Al��OH��3��������������������������������ɣ�����������������ƣ����ⶨ�����ȷ��
�ʴ�Ϊ��Al��OH��3��������������������������������ɣ�����������������ƣ���
��2���ȼ������ܽ������Ȼ������Ȼ�þ��Һ����������������Һ�õ�������þ������
�ʴ�Ϊ��Mg��OH��2��
��3���������������ɵ���ҺΪƫ��������Һ��������A���ʴ����������ﵽʵ��Ŀ�����ͨ�����������̼����������������������̼�����ƣ�NaAlO2+H2O+CO2=Al��OH��3��+NaHCO3��
�ʴ�Ϊ��CO2��NaAlO2+H2O+CO2=Al��OH��3��+NaHCO3��
���� ���⿼�����ʺ����IJⶨ����ʵ��ԭ����װ�õ����⡢ʵ�鷽����Ƶȣ��Ѷ��еȣ�����ʵ��ԭ���ǽ���Ĺؼ����Ƕ�֪ʶ���ۺϿ��飬��Ҫѧ������֪ʶ�Ļ������ۺ�����֪ʶ�������⡢��������������

| A�� | ҪʹAlCl3��Һ��Al3+ȫ������������ʹ�ð�ˮ | |
| B�� | Ҫ����ij��Һ���Ƿ�Fe3+�ɼ������� | |
| C�� | ���Ƶ���ˮ�м���п���ɼ������к�H+ | |
| D�� | ij������ʹƷ����Һ��ɫ��������һ��ΪSO2 |
| A�� | Xһ��ֻ��CO��� | |
| B�� | Xһ��ֻ��H2��CO2��� | |
| C�� | X������0.1 g H2��4.4 g CO2��� | |
| D�� | X������0.1 g H2��1.4 g CO��2.2 g CO2��� |
| A�� | A3BC2 | B�� | A4B2C | C�� | A8B3C3 | D�� | A4B2C2 |
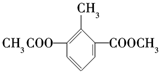
| A�� | һ���������ܷ����ӳɷ�Ӧ��ȡ����Ӧ | |
| B�� | ����ʽ��C11H12O4 | |
| C�� | �����ϵ�һ�ȴ�����2�� | |
| D�� | ����һ�ֺ��������� |
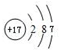 �����������Ļ�ѧʽΪ��Cl2O7��
�����������Ļ�ѧʽΪ��Cl2O7��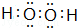 ��
�� ��
��