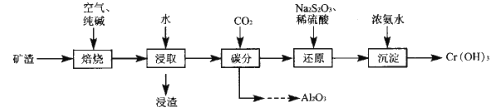
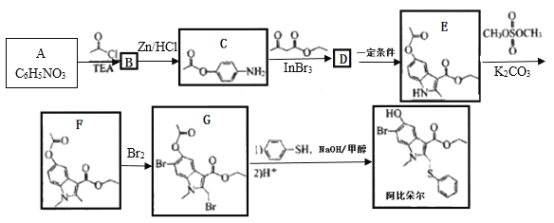
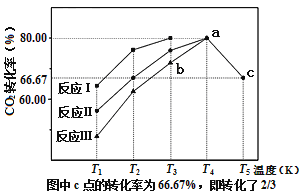
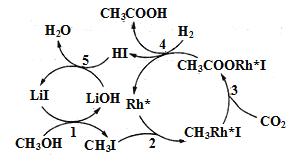
��Ŀ����
����Ŀ��A��B��C��D��E��F��G��Ϊ�л������A����������һ������ʯ�ͻ�����չˮƽ�ı�־�����ʣ�����֮��������ת����ϵ��
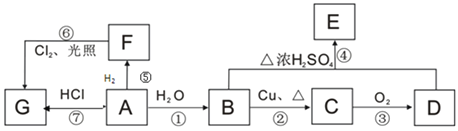
��֪��![]() ����ش��������⣺
����ش��������⣺
(1)B��D�й����ŵ����ƣ�B____________��D________________��
(2)ָ�����б�Ŷ�Ӧ��Ӧ�ķ�Ӧ���ͣ���____________����_____________��
(3)��F��ͬϵ��������л���Ŀռ乹��Ϊ___________������ʽΪ____________��
(4)д����E������ͬ�����ţ�����E��������ͬ���칹��Ľṹ��ʽ��_________��
(5)д�����б�Ŷ�Ӧ��Ӧ�Ļ�ѧ��Ӧ����ʽ��
��_______________________��
��______________________��
���𰸡��ǻ� �Ȼ� �ӳɷ�Ӧ ȡ����Ӧ �������� 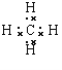 HCOOCH2CH2CH3��HCOOCH(CH3)2��CH3CH2COOCH3 2CH3CH2OH+O2
HCOOCH2CH2CH3��HCOOCH(CH3)2��CH3CH2COOCH3 2CH3CH2OH+O2![]() 2CH3CHO+2H2O CH3COOH+CH3CH2OH
2CH3CHO+2H2O CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O
��������
A����������һ������ʯ�ͻ�����չˮƽ�ı�־�����ʣ���AΪCH2=CH2����ϩ��ˮ�����ӳɷ�Ӧ����BΪCH3CH2OH���Ҵ���������������CΪCH3CHO����ȩ��һ������������Ӧ����DΪCH3COOH���������Ҵ�����������Ӧ����EΪCH3COOCH2CH3����ϩ�����������ӳɷ�Ӧ����FΪCH3CH3����ϩ��HCl��������GΪCH3CH2Cl����������������ȡ����Ӧ���������飬�Դ˽����⡣
��������������֪��AΪCH2=CH2��BΪCH3CH2OH��CΪCH3CHO��DΪCH3COOH��EΪCH3COOCH2CH3��FΪCH3CH3��GΪCH3CH2Cl��
(1)BΪCH3CH2OH�����й�����Ϊ���ǻ���DΪCH3COOH�����й�����Ϊ���Ȼ���
(2)��Ӧ������ϩCH2=CH2��H2O�����ӳɷ�Ӧ�����Ҵ�CH3CH2OH�����Է�Ӧ�ٵ�����Ϊ�ӳɷ�Ӧ����Ӧ����CH3CH3��Cl2�ڹ��������·���ȡ����Ӧ����CH3CH2Cl��HCl�����Է�Ӧ��������ȡ����Ӧ��
(3)F������CH3CH3��������������������F��ͬϵ��������л���Ϊ���飬�ռ乹��Ϊ�������壬����ʽΪ�� ��
��
(4)����E�������������ṹ��ʽΪCH3COOCH2CH3����E������ͬ������(����E)������ͬ���칹��Ľṹ��ʽ��HCOOCH2CH2CH3��HCOOCH(CH3)2��CH3CH2COOCH3��
(5)��Ӧ�����Ҵ�������������Ӧ������ȩ����Ӧ����ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O����Ӧ�����������Ҵ�����������Ӧ����������������Ӧ����ʽΪCH3COOH+CH3CH2OH
2CH3CHO+2H2O����Ӧ�����������Ҵ�����������Ӧ����������������Ӧ����ʽΪCH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��

 ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�����Ŀ��Ϊ̽��H2O2��SO2��Br2������ǿ����ijС��ͬѧ�������ʵ�飨�гּ�β������װ������ȥ���������Ѽ��飩��

ʵ����� | ʵ������ |
��.��A�з�Һ©���������μ�Ũ���� | A�������ݲ�����B�к���ɫ��ˮ��ɫ��C���а�ɫ���� |
��.ȡC�г����������� | C�а�ɫ�������ܽ� |
��.��B�з���©����������εμ�H2O2 | ��ʼʱ��ɫ�����Ա仯�������μ�H2O2��Һ��һ��ʱ����Һ��ɺ���ɫ |
��1��A�з�����Ӧ�Ļ�ѧ����ʽ��____��
��2����ͬѧͨ��C�в�����ɫ�������ó����ۣ������ԣ�H2O2>SO2��
����ͬѧ��Ϊ���ܵó��˽��ۣ���Ϊ�ڵμ�Ũ����֮ǰӦ����һ���������ò�����____��
�ڱ�ͬѧ��Ϊ��Ӧ����B��C֮������ϴ��ƿD��D��ʢ�ŵ��Լ���___��
�۽��Һͱ�ͬѧ�Ľ���ķ�������ʵ�飬C�в�����ɫ�������ó����ۣ�������H2O2>SO2��
��3�����е�������H2O2û�����Ա仯��������裺
�۵�1��H2O2�����ٲ�������Br�D
�۵�2��B����δ��Ӧ��H2SO3
Ϊ��֤�۵�2��Ӧ���е�ʵ�������������________��
��4��ͨ������ȫ��ʵ�飬�ó����ۣ�H2O2��SO2��Br2��������ǿ������˳����____��
����Ŀ����֪��Na2S2O3+H2SO4=Na2SO4+SO2��+S��+H2O��ijͬѧ̽��������������ᷴӦ���ʵ�Ӱ�����أ������һϵ������ʵ�飺
ʵ�� | ��Ӧ�¶�/�� | Na2S2O3��Һ | ϡH2SO4 | H2O | ||
V/mL | c/��molL-1�� | V/mL | c/��molL-1�� | V/mL | ||
A | 10 | 5 | 0.1 | 5 | 0.1 | 5 |
B | 10 | 5 | 0.1 | 5 | 0.1 | 10 |
C | 30 | 5 | 0.1 | 5 | 0.1 | 5 |
D | 30 | 5 | 0.2 | 5 | 0.2 | 10 |
(1)ʵ�������Ϊʲô���ò���һ��ʱ���ڷų�SO2�����������ʾ�÷�Ӧ�Ļ�ѧ��Ӧ���ʴ�С________________________��
(2)��Ҫ̽���¶ȶԷ�Ӧ���ʵ�Ӱ�죬����ѡ��ʵ���е���Щ������Ƚ�_______������ţ���
(3)���÷�Ӧ��I2+2Na2S2O3=Na2S4O6+2NaI�ɶ����ⶨ������������ƵĴ��ȣ��ֳ���������ƹ�����Ʒ�����Һ��ȡһ������Һ������ƿ�У�����ָʾ�����õ�ˮ�ζ����ش����и��⣺
��ʵ���еζ���Ӧѡ��_________������ʽ���ʽ���ζ��ܣ��ζ�ǰ����ѡ��_____��Ϊָʾ�����ζ��յ�ʱ������Ϊ_________________��
�����в��������������Ƶĺ���ƫ�������__________��
A.�ζ�ǰ�ζ����������ݣ��ζ���������ʧ
B.��ȡ��ˮ����̶�ʱ���ζ�ǰƽ�ӣ��ζ�����
C.�ζ������У���ƿ���ھ��ң���������Һ����
D.�ζ���������ˮϴ����ֱ�Ӽ����ˮ��Һ