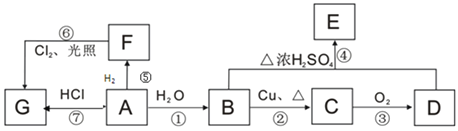
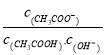��Ŀ����
����Ŀ�����ȷ�ұ���������Ŀ����к���Cr2O7��Al2O3������Fe2O3��������ȡ���������ᷨ�ͼ���ֹ��ա���ش�
I���ᷨ�������������ȡ��ȡҺͨ��������õ�����Cr����ʣ����Һ�мӼ���յõ�Al(OH)3��
(1)Ϊ��߿����Ľ�ȡ�ʣ��ɲ�ȡ�Ĵ�ʩ��_____(д������)��
(2)�����ʱ�������ĵ缫��ӦʽΪ______________��
II. ��������������£�
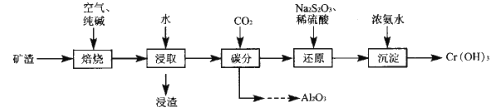
��֪�����������������ɷ�ΪNa2CrO4��NaAlO2��NaFeO2��
��![]() ��Zn2+������EDTA1��1��ϳ������ӣ�Zn2+����PAN1��1��ϳ��Ϻ�ɫ�����ҽ����������EDTA��
��Zn2+������EDTA1��1��ϳ������ӣ�Zn2+����PAN1��1��ϳ��Ϻ�ɫ�����ҽ����������EDTA��
(3)��������Ҫ�ɷ�ΪFe(OH)3��������ȡ��ʱ������Ӧ�����ӷ���ʽΪ_________��
(4)����ȡ����������Һ��Al�ĺ�������EDTA�ζ����ⶨ��
��ȡ20.00mL��ȡҺ����ƿ�У�����c1molL-1EDTA��ҺV1mL(�Թ���)��
�����������ᡢ�����������Ỻ����Һ����Һ�����ԣ����Ⱥ����PANָʾ����
����c2molL-1ZnSO4��Һ�ζ�����Һǡ�ó��Ϻ�ɫ�����ı�ҺV2mL��������ȡ����������Һ��Al�ĺ���Ϊ_________gL-1(�����ʽ����)��
(5)��̼����ʱͨ��CO2��ͨ��_____ (���������)�����ɵõ�������Al2O3��
(6)����ԭ��ʱ������Ҫ��Ӧ�����ӷ���ʽΪ__________��
(7)��������ʱ����c(Cr3+)��10-5molL-1ʱ��Ӧ������Һ��pH����Ϊ_________����Ksp[Cr(OH)3]=1.0��10-32��
���𰸡��ʵ���������Ũ�ȡ����߷�Ӧ�¶ȡ���С�������������ӽ�ȡʱ�䡢����(��������) Cr3++3e- = Cr ![]() +2H2O= Fe(OH)3��+OH-
+2H2O= Fe(OH)3��+OH- ![]() ���ˡ�ϴ�ӡ����� 8
���ˡ�ϴ�ӡ����� 8![]() +3
+3![]() +34H+=6
+34H+=6![]() +8Cr3++17H2O��4
+8Cr3++17H2O��4![]() +3
+3![]() +26H+=6
+26H+=6![]() +8Cr3++13H2O 5
+8Cr3++13H2O 5
��������
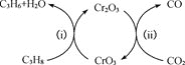
��.��Ͻ�ȡ���ʵ�Ӱ�����ط��������ݵ���ԭ���������
��.�������Ϣ�����ݹ������̷�����֪�������м��봿���ڿ����б�������Na2CrO4��NaAlO2��NaFeO2���ټ�ˮ��ȡ��������Ӧ![]() +2H2O=Fe(OH)3��+OH-������ΪFe(OH)3�����ȡҺ��ͨ��CO2��������Ӧ2H2O+NaAlO2+CO2=NaHCO3+Al(OH)3�������˺���Һ�к���Na2CrO4��������Һ�м���Na2S2O3��ϡ���ᣬNa2CrO4����ԭΪCr3+���ټ��백ˮ�����ɵõ�Cr(OH)3���ݴ˷������
+2H2O=Fe(OH)3��+OH-������ΪFe(OH)3�����ȡҺ��ͨ��CO2��������Ӧ2H2O+NaAlO2+CO2=NaHCO3+Al(OH)3�������˺���Һ�к���Na2CrO4��������Һ�м���Na2S2O3��ϡ���ᣬNa2CrO4����ԭΪCr3+���ټ��백ˮ�����ɵõ�Cr(OH)3���ݴ˷������
��.(1)���ʱ���ɲ����ʵ���������Ũ�ȡ����߷�Ӧ�¶ȡ���С�������������ӽ�ȡʱ�䡢����ȷ�����߿����Ľ�ȡ�ʣ��ʴ�Ϊ���ʵ���������Ũ�ȡ����߷�Ӧ�¶ȡ���С�������������ӽ�ȡʱ�䡢����(��������)��
(2)�����ʱ��C3+�������õ���������Cr���缫��ӦʽΪCr3++3e- =Cr���ʴ�Ϊ��Cr3++3e- =Cr��
��.(3)��������������֪����ˮ��ȡ��������Ӧ![]() +2H2O=Fe(OH)3��+OH-������ΪFe(OH)3���ʴ�Ϊ��
+2H2O=Fe(OH)3��+OH-������ΪFe(OH)3���ʴ�Ϊ��![]() +2H2O=Fe(OH)3��+OH-��
+2H2O=Fe(OH)3��+OH-��
(4)��֪![]() ��Zn2+������EDTA��1��1��ϳ������ӣ�Zn2+����PAN1��1��ϳ��Ϻ�ɫ�����ҽ����������EDTA����
��Zn2+������EDTA��1��1��ϳ������ӣ�Zn2+����PAN1��1��ϳ��Ϻ�ɫ�����ҽ����������EDTA����![]() ���ĵ�EDTA�����ʵ���Ϊ(c1V1-c2V2)��10-3mol����Al�����ʵ���Ϊ(c1V1-c2V2)��10-3mol��������Ϊ27g/mol��(c1V1-c2V2)��10-3mol=27(c1V1-c2V2)��10-3g������Һ��Al�ĺ���Ϊ
���ĵ�EDTA�����ʵ���Ϊ(c1V1-c2V2)��10-3mol����Al�����ʵ���Ϊ(c1V1-c2V2)��10-3mol��������Ϊ27g/mol��(c1V1-c2V2)��10-3mol=27(c1V1-c2V2)��10-3g������Һ��Al�ĺ���Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(5)��̼��/span>��ʱ�����ȡҺ��ͨ��CO2��������Ӧ2H2O+NaAlO2+CO2=NaHCO3+Al(OH)3�������˵õ�Al(OH)3������������ϴ�Ӻ���ȿ�ֱ�ӷֽ�õ�������Al2O3���ʴ�Ϊ�����ˡ�ϴ�ӡ����ȣ�
(6)���˺���Һ�к���Na2CrO4��������Һ�м���Na2S2O3��ϡ���ᣬNa2CrO4����ԭΪCr3+������������ԭ��Ӧ��ʧ�����غ���ɿɵã���Ӧ�����ӷ���ʽΪ8![]() +3
+3![]() +34H+=6
+34H+=6![]() +8Cr3++17H2O��4
+8Cr3++17H2O��4![]() +3
+3![]() +26H+=6
+26H+=6![]() +8Cr3++13H2O���ʴ�Ϊ��8
+8Cr3++13H2O���ʴ�Ϊ��8![]() +3
+3![]() +34H+=6
+34H+=6![]() +8Cr3++17H2O��4
+8Cr3++17H2O��4![]() +3
+3![]() +26H+=6
+26H+=6![]() +8Cr3++13H2O��
+8Cr3++13H2O��
(7)���ݳ����ܽ�ƽ��Cr(OH)3![]() Cr3++3OH-�ɵã�Ksp[Cr(OH)3]=c(Cr3+)��c3(OH-)����c(Cr3+)=10-5molL-1ʱ��
Cr3++3OH-�ɵã�Ksp[Cr(OH)3]=c(Cr3+)��c3(OH-)����c(Cr3+)=10-5molL-1ʱ�� ����pOH=-lg[c(OH-)]=9��pH=14-pOH=5������Ӧ������Һ��pH����Ϊ5���ʴ�Ϊ��5��
����pOH=-lg[c(OH-)]=9��pH=14-pOH=5������Ӧ������Һ��pH����Ϊ5���ʴ�Ϊ��5��

����Ŀ����������������Ҫ�ľ�ϸ�����Լ�������������ˮ����ʳ���㾫��ʵ�����Ʊ�������ͼ��
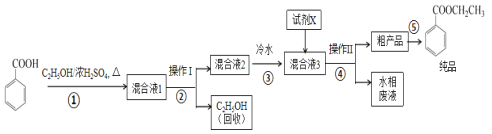
�Լ�����������±���
������ | �Ҵ� | ���������� | |
������״ | ��ɫ��״���� | ��ɫҺ�� | ��ɫ��Һ�� |
�е�/�� | 249.0 | 78.0 | 212.6 |
��Է����� | 122 | 46 | 150 |
�ܽ��� | ����ˮ���������Ҵ������ѵ��л��ܼ� | ��ˮ����Ȼ��� | ��������ˮ��������ˮ���������Ҵ������� |
�ش��������⣺
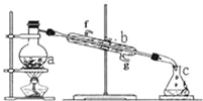
(1)Ϊ���ԭ�ϱ�����Ĵ��ȣ��ɲ��õĴ�������Ϊ__��
(2)����ٵ�װ����ͼ��ʾ(���Ⱥͼг�װ������ȥ)����һС������������B�п��������״�������ˮ��(��ˮ����ͭ���Ҵ�������Һ)��������B�У�������C�м���12.2g������ı����ᾧ�壬30mL��ˮ�Ҵ�(Լ0.5mol)��3mLŨ���ᣬ�����ʯ���������У�������Ӧ1.5��2h������A��������__��
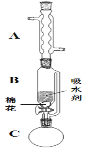
(3)���ŷ�Ӧ���У���Ӧ��ϵ��ˮ�ֲ��ϱ���Ч���룬����B����ˮ��������Ϊ__��
(4)��Ӧ������C�л��Һ���з����ᴿ������I��_������II���õIJ������������ձ����__��
(5)��Ӧ����������н���ӦҺ������ˮ��Ŀ�ij����ܽ��Ҵ��⣬����__�������Լ�XΪ___(��д��ѧʽ)��
(6)���յõ����﴿Ʒ10.0g��ʵ�����Ϊ__%(������λ��Ч����)��
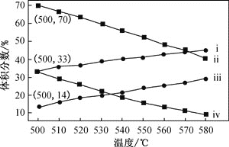
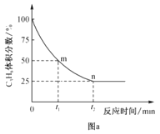
����Ŀ�����û�ѧ��Ӧԭ�����й�֪ʶ�ش��������⣺
(1)�������������ȼ�յ��Ȼ�ѧ����ʽΪSi(s)+O2(g)=SiO2(s)��H=-989.2 kJ��mol-1���йؼ����������±���
��ѧ�� | Si-O | O=O | Si-Si |
����kJ��mol-1 | X | 498.8 | 176 |
��X��ֵΪ_________��
(2)����N2O5�����η����ķֽⷴӦΪ��N2O5![]() N2O3+O2����N2O3
N2O3+O2����N2O3![]() N2O+O2����1 L�ܱ������г���4 mol N2O5�����ȵ�t �棬�ﵽƽ��״̬��O2��ƽ��Ũ��Ϊ4.5 mol/L��N2O3��ƽ��Ũ��Ϊ1.7 mol/L����t��ʱ��Ӧ�ٵ�ƽ�ⳣ��Ϊ_________��
N2O+O2����1 L�ܱ������г���4 mol N2O5�����ȵ�t �棬�ﵽƽ��״̬��O2��ƽ��Ũ��Ϊ4.5 mol/L��N2O3��ƽ��Ũ��Ϊ1.7 mol/L����t��ʱ��Ӧ�ٵ�ƽ�ⳣ��Ϊ_________��