��Ŀ����
����Ŀ�����ҵʼ�� 18 ����ĩ�������������ٶ��ꡣ�ڴ��ҵʷ�ϣ�����ʱ������ά���й��˺�°�ȶ�������ͻ���Ĺ��ס��ݴ��������С�⡣
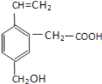
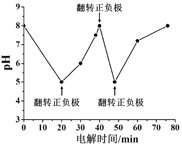
��1����ͼ��1861������ά�������Ƽ������ת����ϵͼ������I~IV�����ĸ���Ҫ��ѧ��Ӧ��a~i������H2O���ⲻͬ�����ʡ�������������ģ�������˵����ȷ���ǣ� ��
A.ˮ��Һ�ʼ��Ե�����ֻ��e��g��h
B.ˮ��Һ�����Ե�����ֻ��c��f��i
C.ÿ����1mol a��ͬʱ����0.5mol b
D.ÿ����1mol h��ͬʱ����0.5mol i
��2����ĸҺ��������NaHCO3�к���NH4Cl����ϴ�Ӻ��ٽ������ա���ʡ��ϴ�Ӳ����������ն����ô����Ӱ��������ȷ���ǣ� ��
A.������Ӱ��B.NH4Cl���ʺ�������
C.NaCl���ʺ�������D.NaOH���ʺ�������
��3������ר�Һ�°�����"�����Ƽ"����ƽ��˴��ҵ�ķ�չ��������Ҫ�����ǣ� ��
A.�ҵ�������Ч�Ĵ���B.�������������
C.����˴����Ʒ�Ĵ���D.������Na+��������
���𰸡�
��1��C
��2��C
��3��D
��������
��1�������ʳ��(�Ȼ���)��ʯ��ʯ(������������ʯ�ҺͶ�����̼)������Ϊԭ������ȡ�����ʹ����ͨ�뱥��ʳ��ˮ�ж��ɰ���ˮ����ͨ�������̼�����ܽ�Ƚ�С��̼�����Ƴ������Ȼ����Һ���������ˡ�ϴ�ӵõ���NaHCO3С�����ټ��������Ƶô����Ʒ���ų��Ķ�����̼����ɻ���ѭ��ʹ�ã������Ȼ�淋���Һ��ʯ����[Ca(OH)2]��ϼ��ȣ����ų��İ����ɻ���ѭ��ʹ�ã�������ת����ϵͼ��֪aΪNaCl��bΪCaCO3��cΪCO2��dΪNaHCO3��eΪNH3��fΪNH4Cl��gΪCaO��hΪNa2CO3��iΪCaCl2��
A��ˮ��Һ�ʼ��Ե�������NaHCO3��NH3��CaO��Na2CO3����A����
B��ˮ��Һ�����Ե�������CO2��NH4Cl����B����
C����Na2CO3��Ԫ���غ��֪��n(Na)=2n(C)����n(NaCl)=n(Na)��n(C)=n(CaCO3)���ɵ�n(NaCl)=2 n(CaCO3)����ÿ����1molNaCl��ͬʱ����0.5mol CaCO3����C��ȷ��
D��������ת����ϵͼ��֪h�� i���������û�б����ģ���D����
�ʴ�ѡC��
��2����ĸҺ��������NaHCO3�к���NH4Cl����ʡ��ϴ�Ӳ�����NH4Clˮ�������HCl���NaHCO3��Ӧ����NaCl��ʹNaCl���ʺ����������ʴ�ѡC��
��3���������Ƽ������ڰ�����Ȼ��������ʴ�70%��ߵ�90%���ϣ�����������Ҫ������������Na+�������ʣ��ʴ�ѡD��

 ���ƿ�����ϵ�д�
���ƿ�����ϵ�д� ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�����Ŀ��ij������Ʒ�к��������Ȼ��ƣ������ⶨ��̼���Ƶ������������������ʵ�鷽����
������1����ȡһ�������Ĵ�����Ʒ����֪��ƿ��������Һ������190.720 g����������ͼװ�òⶨ������Ʒ�Ĵ��ȣ�ÿ����ͬʱ����õ�����ƽ�����������
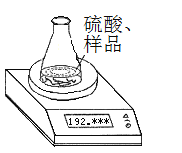
�������� | ������g�� | |
��ƿ+����+���� | ��1�� | 192.955 |
��2�� | 192.764 | |
��3�� | 192.328 | |
��4�� | 192.075 | |
��5�� | 192.075 |
��1�����㴿����Ʒ�Ĵ���ʱ�������������_____________________________����������ݣ�����������6�ζ�����ԭ����________________________________________________��
��2�����㴿����Ʒ�Ĵ���Ϊ_________________________������С������λ����
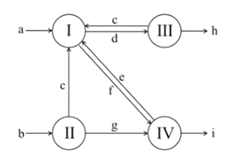
������2���ⶨ������Ʒ��1.15 g���У�Na2CO3������������һ�ַ�����������������£�
��1����Һת����__________����д�������ƣ�������II��������______________��
��2����ֱ�Ӳⶨ����������____________________��
��3���ⶨ��������Ҫ�������е�����ƽ�������ƾ��ơ�����Ҫ__________��__________���̶����г��������⣩��
��4����ת����Һʱ������Һת�Ʋ���ȫ����Na2CO3���������IJⶨ���__________������ƫС������ƫ����������������
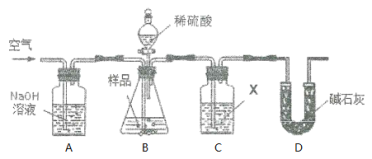
������3��ʵ��װ����ͼ��
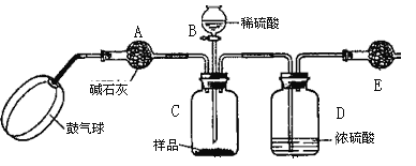
ʵ�鲽�裺
����ͼ����װ�ò���������ҩƷ��
�ڳ�������¼E������m1������ʱע����E�����ˣ���
�۰�����������Լ1���ӡ�
��������E��
�ݴ�Һ©��B�Ļ�������ϡ������ټ���C�кرջ�����
�ް�����������Լ1���ӡ�
�߳�������¼E������m2������ʱע����E�����˼�D�Ҷ˵ij��ڣ���
���ظ�����͢ߵIJ�����ֱ�����θ���ܵ������������䣬��Ϊm3��
����㡣
����պͻش����⣺
��1��C�з�����Ӧ�����ӷ���ʽΪ��______________________________________��B����������Ϊ__________���������Һ©���е����ỻ��Ũ����ͬ�����ᣬ���ԵĽ����__________������ƫ��������ƫ������������������
��2��Ũ�����������_________________����û��D����ʵ����__________������ƫ��������ƫ����������Ӱ��������
��3������ۺ͢�������____________________��____________________����������ٶ��ǿ��ٺã����ǻ������룿Ϊʲô��__________________________________________��
��4��Eװ�õĹ����Լ�Ϊ__________�����ţ�
A����ʯ�� B����ˮ�Ȼ��� C��Ũ���� D����ʯ��
��5��������Ŀ����________________________________________________��
��6�������д�����Ʒ��������������ʽΪ_____________________________________��
��7����ʵ�������������Ҫ�Ľ��ĵط�����ָ���ý�֮����˵��ԭ��
_______________________________________________________________________��
��8��ʵ�黹��������������ʵ�鷽���ⶨ�����д�������������������һ�ֲ�ͬ�Ķ���ʵ�鷽����___________________________________________________________ ��