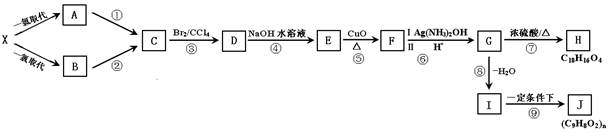
��Ŀ����
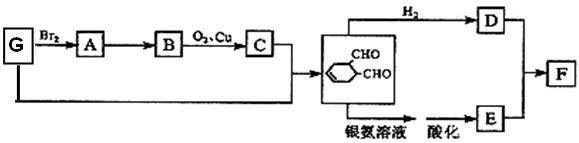
��10�֣�ú��������Һ����ʹú��������Դ����Ч;����ú��������Ҫ��Ӧ�ǣ�C+H2O(g)����CO+H2��CO��H2�Ļ�������Ǻϳɶ����л����ԭ��������ͼ�Ǻϳ�ijЩ���ʵ�·�ߡ����У�D������ˮ������CH3COOH��Ϊͬ���칹�壬������������Һ��Ӧ�����ܷ���������Ӧ��F�����е�̼ԭ������D��3����H���������ɵõ�G����ش��������⣺
��1��д���������ʵĽṹ��ʽ��E___________B__________��E��H�Ƿ�����ͬϵ�________________�����ǻ��
��2��D��ͬ���칹���CH3COOH��У�___________________��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
C+H_____________________________________________��
D��������Һ��Ӧ________________________________________��
��4��AΪ��ҵ����ȼ�ϡ�ijͬѧ��A��ȫȼ�գ������Ķ�����̼����ͨ�����ʯ��ˮ�С�
����ͬѧʵ��ʱ��ȡ��200mL0.1mol/L��ʯ��ˮ��ͨ��һ�����Ķ�����̼�õ�1g��������ô����ͨ��Ķ�����̼����µ��������Ϊ___________________________��

��1��д���������ʵĽṹ��ʽ��E___________B__________��E��H�Ƿ�����ͬϵ�________________�����ǻ��
��2��D��ͬ���칹���CH3COOH��У�___________________��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
C+H_____________________________________________��
D��������Һ��Ӧ________________________________________��
��4��AΪ��ҵ����ȼ�ϡ�ijͬѧ��A��ȫȼ�գ������Ķ�����̼����ͨ�����ʯ��ˮ�С�
����ͬѧʵ��ʱ��ȡ��200mL0.1mol/L��ʯ��ˮ��ͨ��һ�����Ķ�����̼�õ�1g��������ô����ͨ��Ķ�����̼����µ��������Ϊ___________________________��
��1��HOCH2CH2OH ��1�֣� HCHO ��1�֣� ��1�֣���2��HCOOCH3��1�֣���3��

��4��0.224L��0.672L��2�֣�


��4��0.224L��0.672L��2�֣�
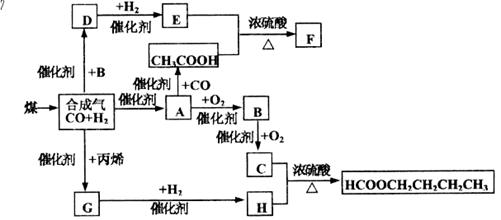
ͻ�ƿڣ�D��HOCH2CHO���������ӳ�����EΪHOCH2CH2OH,E��������������F��
��һͻ�ƿڣ���HCOOCH2CH2CH2CH3���ƣ�HΪHOCH2CH2CH2CH3��CΪHCOOH��BΪHCHO��AΪCH3OH,��H����GΪCH3CH2CH2CHO
��4��n[Ca(OH)2]=0.02mol,���ɵ�n(CaCO3)=0.01molȻ����������������
�ٶ�����̼����
Ca(OH)2+CO2=CaCO3��+H2O
1 1
0.01mol 0.01mol ��V��CO2��=0.224L
�ڶ�����̼������ �� Ca(OH)2+CO2=CaCO3��+H2O �� CaCO3+ CO2+ H2O =Ca(HCO3)2
1 1 1 1 1
0.02mol 0.02mol0.02mol 0.01mol 0.01mol
��V��CO2��=��0.02mol+0.01mol����22.4mol.L-1=0.672L
��һͻ�ƿڣ���HCOOCH2CH2CH2CH3���ƣ�HΪHOCH2CH2CH2CH3��CΪHCOOH��BΪHCHO��AΪCH3OH,��H����GΪCH3CH2CH2CHO
��4��n[Ca(OH)2]=0.02mol,���ɵ�n(CaCO3)=0.01molȻ����������������
�ٶ�����̼����
Ca(OH)2+CO2=CaCO3��+H2O
1 1
0.01mol 0.01mol ��V��CO2��=0.224L
�ڶ�����̼������ �� Ca(OH)2+CO2=CaCO3��+H2O �� CaCO3+ CO2+ H2O =Ca(HCO3)2
1 1 1 1 1
0.02mol 0.02mol0.02mol 0.01mol 0.01mol
��V��CO2��=��0.02mol+0.01mol����22.4mol.L-1=0.672L

��ϰ��ϵ�д�
 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�
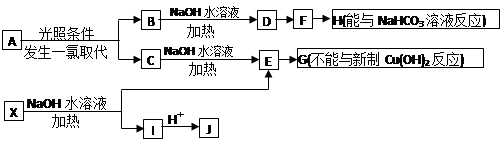
�����Ŀ
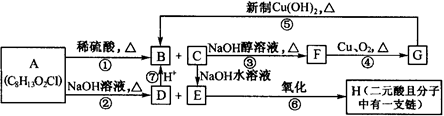
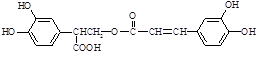 �����Ǵ���������ֲ���е�һ�ֶ�ӣ����п��������ӻ�˥�ϡ����ʽ�֬�ȹ�Ч����AΪԭ�Ϻϳ�F��·�����£�
�����Ǵ���������ֲ���е�һ�ֶ�ӣ����п��������ӻ�˥�ϡ����ʽ�֬�ȹ�Ч����AΪԭ�Ϻϳ�F��·�����£�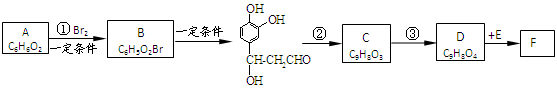
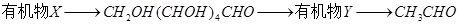

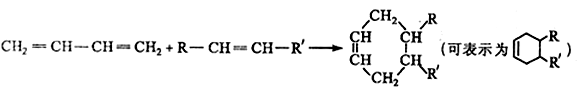
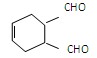 �й���������Ϊ ��
�й���������Ϊ ��
 ��
��