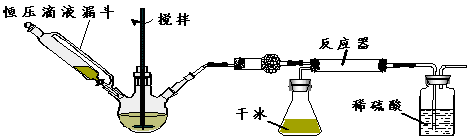
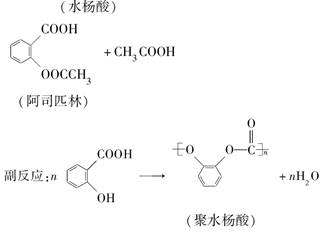
��Ŀ����
����������Ҫ�ľ�ϸ����ԭ��,��ҽҩ��Ⱦ�ϵ��м��壬�������л��ܼ����Ʊ��������Ĺ������£������ƻ����װ����ͼ��Ӧװ�á�
ȡ100 mL�ձ�����20 mLŨ������Ũ����18 mL���ƻ���ᣬ����©���С���18 mL������������ƿ�С�
���������µı�����μ�����ᣬ�ߵα߽��裬��;��ȡ�
����50-60���·�����Ӧ��ֱ����Ӧ������
�ܳ�ȥ�����ֲ�Ʒ����������ˮ��10%Na2CO3��Һϴ�ӣ������������ˮϴ�ӵõ��ֲ�Ʒ��
��֪��1��
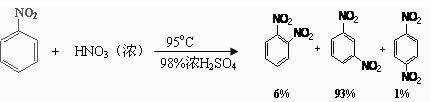
��2�������õ����й������б�����
| ���� | �۵�/�� | �е�/�� | �ܶ�(20 ��) / g��cm-3 | �ܽ��� |
| �� | 5.5 | 80 | 0.88 | ����ˮ |
| ������ | 5.7 | 210.9 | 1.205 | ������ˮ |
| 1,3-�������� | 89 | 301 | 1.57 | ����ˮ |
| Ũ���� |  | 83 | 1.4 | ������ˮ |
| Ũ���� |  | 338 | 1.84 | ������ˮ |
��ش��������⣺
��1�����û���Ӧ�����ձ����ȼ��� ��
��2����ѹ��Һ©�����ŵ��� ��
��3��ʵ��װ���г������ܿ��� ���棨���������ƣ���
��4����Ӧ�������Ʒ��Һ��� �㣨��ϡ����ߡ��¡������������Ͳ�Ʒ�IJ�������Ϊ ��
��5����10%Na2CO3��Һϴ��֮����������ˮϴ��ʱ��������֤Һ����ϴ��? ��
��6��Ϊ�˵õ�������������������������Һ���м��� ��ȥˮ��Ȼ������
��1��Ũ����
��2�����Ա���©����ѹǿ�뷢������ѹǿ��ȣ�ʹ©����Һ����˳������
��3�������ܣ����������ܻ�ֱ�������ܾ��ɣ�
��4���� ��Һ
��5��ȡ���һ��ϴ��Һ������Һ�м����Ȼ��ƣ��������ɣ�˵����ϴ����
��6�� �Ȼ���
���������������1������Ũ������ܶȱ�Ũ����Ĵ�Ũ������ˮʱ�ų��������ȣ��������û���Ӧ��Ũ������뵽Ũ�����У�Ҳ���������ձ����ȼ���Ũ���ᡣ��2�������ڷ�Ӧ�Ĺ����в��ϼ��ȣ���������������壬�����Һ��ļ���ܲ����������ú�ѹ��Һ©���μӣ����Ա���©����ѹǿ�뷢������ѹǿ��ȣ�ʹ©����Һ����˳�����¡���3����ʵ��Ĺ����б����������Ϊ���ȶ��������������ʵ��˷��뻷����Ⱦ��������װ���г������ܿ���������������ʹ���ʻ��������á���˿��������ܵ�����װ�ô��档��4����Ӧ�������������������1,3-�������������ܽ���ˮ��Һ�壬�ܶȱ���Ļ����ҺС�����Է�Ӧ�������Ʒ��Һ����ϲ㣬���뻥�����ܵ�����Һ��ķ����Ƿ�Һ����5�����ڵõ��Ĵֲ�Ʒ����������ˮ��10%Na2CO3��Һϴ�ӣ������������ˮϴ�ӡ����ϴ�Ӹɾ�����ϴ��Һ�в�����CO32-�����Լ���Һ����ϴ���ķ�����ȡ���һ��ϴ��Һ������Һ�м����Ȼ��ƣ����������ɣ�˵����ϴ������6��Ϊ�˵õ�����������������ʹ֮������ˮ����������Һ���м�������ˮ�������õ���ˮCaCl2����ȥˮ��Ȼ�����͵õ��˲�Ʒ��
���㣺������Ҫ�ľ�ϸ����ԭ������������ȡ�������漰�����ʵĻ�ϡ������ķ��롢���ʵ�ϴ�ӡ������ʵ�������֪ʶ��

������ʾ��þ�뱥��̼��������Һ��Ӧ������������Ͱ�ɫ�����ijͬѧ���������ʵ�鷽��̽����Ӧԭ������֤���
(1)�������
ʵ�����ɰֽ��ȥþ����������Ĥ���������ʢ���������з�̪�ı���̼��������Һ���Թ��У�Ѹ�ٷ�Ӧ�������������ݺͰ�ɫ�������Һ��dz���졣
��ͬѧ�Է�Ӧ�в����İ�ɫ�������������²²⣺
�²�1����ɫ���������Ϊ________��
�²�2����ɫ���������ΪMgCO3��
�²�3����ɫ����������Ǽ�ʽ̼��þ[xMgCO3��yMg(OH)2]��
(2)��ƶ���ʵ��ȷ�����ﲢ��֤�²⣺
| ʵ����� | ʵ�� | ʵ������ | ���� |
| ʵ��� | ��ʵ������� �����������ȼ | �ܰ���ȼ�ա��� ������ɫ���� | ����ɷ�Ϊ __��__ |
| ʵ��� | ȡʵ����еİ� ɫ�����ϴ�ӣ� ��������__��__ | __��__ | ��ɫ��������ܺ���MgCO3 |
| ʵ��� | ȡʵ����еij� ��Һ�������м��� ����CaCl2ϡ��Һ | ������ɫ���� | ��Һ�д���__��__ |
(3)Ϊ��һ��ȷ��ʵ���IJ����ƶ���ʵ�鷽������ͼ��ʾ��
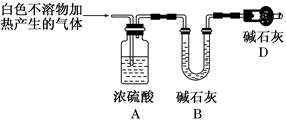
��ȡʵ��������ø�������İ�ɫ������22.6 g����ּ��������ٲ�������Ϊֹ����ʹ�ֽ����������ȫ������װ��A��B�С�ʵ��ǰ��װ��A����1.8 g��װ��B����8.8 g����ȷ����ɫ������Ļ�ѧʽΪ_____________________________________________��
(4)���ϻ�ѧ����ͻ�ѧƽ���ƶ�ԭ������Mg�ͱ���NaHCO3��Һ��Ӧ�����������ݵ�ԭ��______________________________________________________________
________________________________________________________________________��
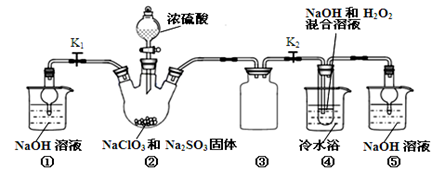
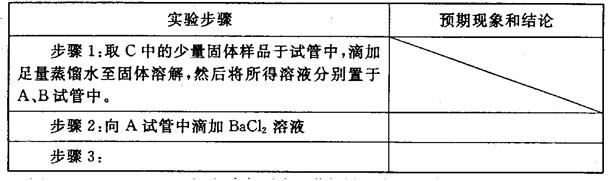
��ij��ѧ�С���������ͼ��ʾ�����ּг�װ������ȥ��ʵ��װ�ã���̽����ʪ��Cl2�����Na2CO3 ���巴Ӧ�õ��Ĺ������ʵijɷ֡�
��֪��ͨ��һ�������������D��ֻ��һ�ֳ�����Ϊ�ƺ�ɫ�����壬��Ϊ�������������ȷ������C�й��庬��NaHCO3 ���Һ��ȵ���ֻ��һ�֡��ֶ�C�ijɷֽ��в����̽����
��1������������룺����֪C����0.1molCl2ǡ�ú�10.6��Na2CO3������ȫ��Ӧ����C����Cl2���뷴Ӧ�Ļ�ѧ����ʽ���� ��
��2��������������衣
����1���������ֳɷ֣�NaHCO3�� ��
����2���������ֳɷ֣�NaHCO3�� �� ��
����ƺ���������C�����е�δ֪�ɷֽ���̽������д��ʵ�鲽���Լ�Ԥ������ͽ��ۣ��ɲ���������
��ѡʵ���Լ�������������ˮ��ϡHNO3��Ba(OH)2��Һ��BaCl2��Һ������ʯ��ˮ��AgNO3��Һ���Թܡ�С�ձ���
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����C�й�����Ʒ���Թ��У�������������ˮ���������������ȫ�ܽ⣬Ȼ��������Һ��װA��B��֧�Թ��С� | |
| ����2�� | |
| ����3�� | |
| | |
�������ձ�������������Ͳ����ͷ�ιܣ�Ҫ����0.0150 mol/L K2Cr2O7��Һ100mL������Ҫ�IJ��������� ��
��ȡ25mL����Һ���еζ���ƽ������ K2Cr2O7��Һ���Ϊ25.00 mL��������ʯ����Ԫ�صİٷֺ����ǣ�Fe�����ԭ������Ϊ56�� ��
���ڱ�ʵ��ĵζ������У����в�����ʹ�ⶨ���ƫС���� ����д��ţ���
a��δ�ñ�K2Cr2O7��Һ��ϴ�ζ���
b����ƿ�м��������Һ���ټ�����ˮ
c����ƿ�ڵζ������о���ҡ����������Һ�彦��
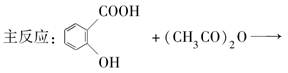



 NH2COONH4(s)���÷�Ӧ�ڸ��������½����ɰ�������泥�����ˮ����������̼��炙�̼����李�
NH2COONH4(s)���÷�Ӧ�ڸ��������½����ɰ�������泥�����ˮ����������̼��炙�̼����李�