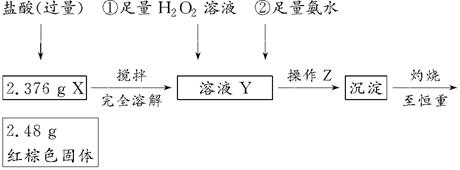
题目内容
资料显示:镁与饱和碳酸氢钠溶液反应产生大量气体和白色不溶物。某同学设计了如下实验方案探究反应原理并验证产物。
(1)提出假设
实验Ⅰ:用砂纸擦去镁条表面氧化膜,将其放入盛有适量滴有酚酞的饱和碳酸氢钠溶液的试管中,迅速反应,产生大量气泡和白色不溶物,溶液由浅红变红。
该同学对反应中产生的白色不溶物做出如下猜测:
猜测1:白色不溶物可能为________;
猜测2:白色不溶物可能为MgCO3;
猜测3:白色不溶物可能是碱式碳酸镁[xMgCO3·yMg(OH)2]。
(2)设计定性实验确定产物并验证猜测:
| 实验序号 | 实验 | 实验现象 | 结论 |
| 实验Ⅱ | 将实验Ⅰ中收 集到的气体点燃 | 能安静燃烧、产 生淡蓝色火焰 | 气体成分为 __①__ |
| 实验Ⅲ | 取实验Ⅰ中的白 色不溶物,洗涤, 加入足量__②__ | __③__ | 白色不溶物可能含有MgCO3 |
| 实验Ⅳ | 取实验Ⅰ中的澄 清液,向其中加入 少量CaCl2稀溶液 | 产生白色沉淀 | 溶液中存在__④__ |
(3)为进一步确定实验Ⅰ的产物,设计定量实验方案,如图所示:

称取实验Ⅰ中所得干燥、纯净的白色不溶物22.6 g,充分加热至不再产生气体为止,并使分解产生的气体全部进入装置A和B中。实验前后装置A增重1.8 g,装置B增重8.8 g,试确定白色不溶物的化学式为_____________________________________________。
(4)请结合化学用语和化学平衡移动原理解释Mg和饱和NaHCO3溶液反应产生大量气泡的原因:______________________________________________________________
________________________________________________________________________。
(1)Mg(OH)2
(2)①氢气 ②稀盐酸(合理均可) ③产生气泡,沉淀全部溶解 ④CO32—
(3)2MgCO3·Mg(OH)2或Mg(OH2)·2MgCO3或Mg3(OH)2(CO3)2
(4)NaHCO3溶液中存在平衡:HCO3— H++CO32—、H2O
H++CO32—、H2O H++OH-。Mg和H+反应生成H2和Mg2+,Mg2+跟OH-、CO32—生成难溶物Mg(OH)2·2MgCO3,则H+、OH-、CO的浓度均降低,促使上述两平衡均向右移动,故Mg和饱和NaHCO3溶液反应产生大量气体H2
H++OH-。Mg和H+反应生成H2和Mg2+,Mg2+跟OH-、CO32—生成难溶物Mg(OH)2·2MgCO3,则H+、OH-、CO的浓度均降低,促使上述两平衡均向右移动,故Mg和饱和NaHCO3溶液反应产生大量气体H2
解析

发酵粉是一种化学膨松剂,可由小苏打、臭粉(碳酸氢铵)、明矾中的两种物质组成。某小组为探究不同品牌的发酵粉的化学成分,进行如下实验。
【提出假设】
(1)假设1:由小苏打和臭粉组成
假设2:由小苏打和明矾组成
假设3:由 组成
【方案与流程】
为探究甲品牌的发酵粉的成分,某同学设计如下实验,得到如下现象: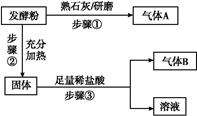
(2)结合步骤①~③分析,气体A为 ,该发酵粉的成分为 (填化学式)。
(3)若步骤①和②操作不变(现象也相同),将步骤③中足量稀盐酸改为足量氯化钡溶液,观察到有白色沉淀生成,能否确定发酵粉的成分并说明理由: 。
(4)乙品牌的发酵粉的化学组成可能为假设2情况,请你设计实验验证。
实验仪器和用品任选,限选试剂:稀盐酸、0.1 mol/L NaOH溶液
写出实验步骤、预期现象和结论。
| 实验步骤 | 预期现象和结论 |
| 步骤1:取少量样品溶于足量的盐酸后,将溶液分成两份,分别装入A、B试管中 | |
| 步骤2: | ,证明有Na+,发酵粉中有NaHCO3 |
| 步骤3: | ,结合步骤2中的结论,假设2成立 |
乙酰水杨酸俗称阿司匹林(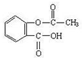 ),是世界上应用最广泛的解热、镇痛和抗炎药。乙酰水杨酸受热易分解,分解温度为128℃~135℃。实验室以水杨酸(邻羟基苯甲酸)与醋酸酐[(CH3CO)2O]为主要原料合成阿司匹林,其制备原理为:
),是世界上应用最广泛的解热、镇痛和抗炎药。乙酰水杨酸受热易分解,分解温度为128℃~135℃。实验室以水杨酸(邻羟基苯甲酸)与醋酸酐[(CH3CO)2O]为主要原料合成阿司匹林,其制备原理为:


制备基本操作流程如下:
主要试剂和产品的物理常数如下表:
| 名称 | 相对分子质量 | 熔点或沸点(℃) | 水 |
| 水杨酸 | 138 | 158(熔点) | 微溶 |
| 醋酸酐 | 102 | 139.4(沸点) | 反应 |
| 乙酰水杨酸 | 180 | 135(熔点) | 微溶 |
回答下列问题:
(1)合成阿司匹林时,最合适的加热方法是 。
(2)合成阿司匹林时,必须使用干燥的仪器,其原因是 。
(3)减压过滤所得粗产品要用少量冰水洗涤,其目的是 。
(4)用重结晶方法提纯粗产品流程如下,加热回流程装置如图。


①沸石的作用是 ;
②冷凝水的流进方向是 (填“a”或“b”);
③使用温度计的目的是 。
(5)在实验中原料用量:2.0g水杨酸、5.0mL醋酸酐(
 ),最终称得产品质量为2.2g,则所得乙酰水杨酸的产率为 (百分数精确到0.1)。
),最终称得产品质量为2.2g,则所得乙酰水杨酸的产率为 (百分数精确到0.1)。 硫化碱法是工业上制备Na2S2O3的方法之一,反应原理为:2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2 (该反应△H>0)
某研究小组在实验室用硫化碱法制备Na2S2O3·5H2O流程如下。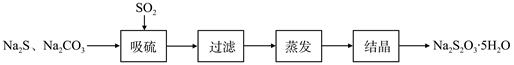
(1)吸硫装置如图所示。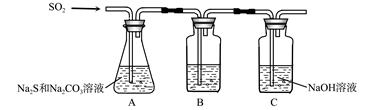
①装置B的作用是检验装置A中SO2的吸收效率,B中试剂是 ,表明SO2吸收效率低的实验现象是B中溶液 。
②为了使SO2尽可能吸收完全,在不改变A中溶液浓度、体积的条件下,除了及时搅拌反应物外,还可采取的合理措施是 、 。(写出两条)
(2)假设本实验所用的Na2CO3含少量NaCl、NaOH,设计实验方案进行检验。(室温时CaCO3饱和溶液的pH=10.2)
限选试剂及仪器:稀硝酸、AgNO3溶液、CaCl2溶液、Ca(NO3)2溶液、酚酞溶液、蒸馏水、pH计、烧杯、试管、滴管
| 序号 | 实验操作 | 预期现象 | 结论 |
| ① | 取少量样品于试管中,加入适量蒸馏水,充分振荡溶解,___________________。 | _______________ | 样品含NaCl |
| ② | 另取少量样品于烧杯中,加入适量蒸馏水,充分搅拌溶解,___________________。 | _______________ | 样品含NaOH |
(3)Na2S2O3溶液是定量实验中的常用试剂,测定其浓度的过程如下:准确称取a g KIO3(化学式量:214)固体配成溶液,加入过量KI固体和H2SO4溶液,滴加指示剂,用Na2S2O3溶液滴定至终点,消耗Na2S2O3溶液的体积为V mL。则c(Na2S2O3)=_________mol·L-1。(只列出算式,不作运算)
已知:IO3-+5I-+6H+== 3I2+3H2O 2S2O32-+I2==S4O62-+2I-
乙二酸(H2C2O4)俗称草酸,是一种重要的化工原料。查阅资料,了解到以下有关信息:
①乙二酸易溶于水,加热至100℃开始升华,125℃时迅速升华,157℃时大量升华并开始分解。乙二酸受热分解生成水、二氧化碳和一种常见的还原性气体。
②乙二酸的钙盐——乙二酸钙为不溶于水的白色晶体。
(1)请写出乙二酸受热分解的化学方程式______________________。
(2)化学兴趣小组的同学用实验证明乙二酸晶体受热分解生成的气体成分。他们利用下图提供的装置,自选试剂,提出了下列实验方案:按A→B→C→C→C→D→E顺序从左至右连接装置,检验乙二酸晶体受热分解生成的气体成分。
请你按整套装置从左至右的顺序填写下表中的空格:
| 仪器符号 | 仪器中所加物质 | 装置作用 |
| B | | |
| C | | |
| C | 氢氧化钠浓溶液 | |
| C | | |
| D | | |
| E | | |
(3)上述实验中能说明乙二酸受热分解生成了还原性气体的实验现象是________________________________________________________________。
(4)检验乙二酸具有较强的还原性,通常选用的试剂是_______________。
硝基苯是重要的精细化工原料,是医药和染料的中间体,还可做有机溶剂。制备硝基苯的过程如下:①配制混酸:组装如下图反应装置。
取100 mL烧杯,用20 mL浓硫酸与浓硝酸18 mL配制混和酸,加入漏斗中。把18 mL苯加入三颈烧瓶中。
②向室温下的苯中逐滴加入混酸,边滴边搅拌,混和均匀。
③在50-60℃下发生反应,直至反应结束。
④除去混和酸后,粗产品依次用蒸馏水和10%Na2CO3溶液洗涤,最后再用蒸馏水洗涤得到粗产品。
已知(1)

(2)可能用到的有关数据列表如下
| 物质 | 熔点/℃ | 沸点/℃ | 密度(20 ℃) / g·cm-3 | 溶解性 |
| 苯 | 5.5 | 80 | 0.88 | 微溶于水 |
| 硝基苯 | 5.7 | 210.9 | 1.205 | 难溶于水 |
| 1,3-二硝基苯 | 89 | 301 | 1.57 | 微溶于水 |
| 浓硝酸 |  | 83 | 1.4 | 易溶于水 |
| 浓硫酸 |  | 338 | 1.84 | 易溶于水 |
请回答下列问题:
(1)配置混酸应先在烧杯中先加入 。
(2)恒压滴液漏斗的优点是 。
(3)实验装置中长玻璃管可用 代替(填仪器名称)。
(4)反应结束后产品在液体的 层(填“上”或者“下”),分离混酸和产品的操作方法为 。
(5)用10%Na2CO3溶液洗涤之后再用蒸馏水洗涤时,怎样验证液体已洗净? 。
(6)为了得到更纯净的硝基苯,还须先向液体中加入 除去水,然后蒸馏,
 )如下:
)如下: (水杨酸)+SOCl2→
(水杨酸)+SOCl2→ (水杨酰氯)+HCl↑+SO2↑
(水杨酰氯)+HCl↑+SO2↑
 ),温度控制在100℃左右,不断搅拌。
),温度控制在100℃左右,不断搅拌。