��Ŀ����
3�� ij��ѧ��ȤС������ͼ��ʾװ�ý��е绯ѧԭ����ʵ��̽�����Իش��������⣺
ij��ѧ��ȤС������ͼ��ʾװ�ý��е绯ѧԭ����ʵ��̽�����Իش��������⣺��1��ͨO2��Pt�缫Ϊ�����������缫���ƣ�����缫����ʽΪO2+4e-+2H2O=4OH-��
��2����B���Ϊ��Ƴأ�Ŀ������ij�Ƽ��϶�һ��������X�缫����ΪAg���������ҺΪAgNO3��Һ��
��3����B���Ϊ����ͭ���Ҵ�ͭ�к���Zn��Fe��Ag��Au�����ʣ���õ�����������Ҫ�ɷ���Ag��Au��
��4����B��صĵ������ҺΪ500mL 1.0mol/L��NaCl��Һ��X��Y��Ϊ���Ե缫������ع���һ��ʱ��Ͽ���ԴK��Y�缫��560mL����״������ɫ�������ɣ�����缫����������ȫ�������Һ������䣩����ʱB�����Һ��pH=13��Ҫʹ����Һ�ָ���ԭ����״̬�������0.05molHCl�������ʲ�ע�����ʵ�������
��5����X��Y����ͭ���������ҺΪNaOH��Һ����ع���һ��ʱ�䣬X����������ש��ɫ�������������ϵ�֪��Cu2O����д���õ缫�����ĵ缫��ӦʽΪ2Cu+2OH--2e-=Cu2O+H2O��
���� ��1�������ȼ�ϵ�أ��ұ��ǵ��أ�ͨ������һ��������������ԭ��Ӧ���缫��ӦʽΪ��O2+4e-+2H2O=4OH-��
��2��X���Դ����������������������������Ӧ���������Һ����������Һ��
��3����ͭ�к���Zn��Fe��Ag��Au�����ʣ�����п�������ڵ缫�Ϸŵ����������ӽ�����Һ���������������Ҫ�ɷ���Ag��Au��
��4����ⱥ��ʳ��ˮ������������ʧ�������������������������ӵõ������������������ݵ��ԭ����������д��ط�Ӧ���������ɵ������������������������Ũ�ȣ�������ӻ���������������Ũ�ȼ�����ҺpH���ָ���ҺŨ�����ݳ�ʲô����ʲô��ԭ�������
��5��X�缫����������������Ӧ���缫��ӦʽΪ��2Cu+2OH--2e-=Cu2O+H2O��
��� �⣺��1��A����ȼ�ϵ�أ�B���ǵ��أ�ͨ������һ��������������ԭ��Ӧ���缫��ӦʽΪ��O2+4e-+2H2O=4OH-���ʴ�Ϊ������O2+4e-+2H2O=4OH-��
��2��X���Դ����������������������������Ӧ������X�缫����Ϊ�����������Һ����������Һ���ʴ�Ϊ��Ag��AgNO3��Һ��
��3����ͭ�к���Zn��Fe��Ag��Au�����ʣ�����п�������ڵ缫�Ϸŵ����������ӽ�����Һ���������������Ҫ�ɷ���Ag��Au���ʴ�Ϊ��Ag��Au��
��4��Y�缫��ԭ��صĸ����������缫�����ӵõ�����������������������560ml�����ʵ���=$\frac{0.56L}{22.4L/mol}$=0.025mol�����ݵ�ⷽ��ʽ��
2Cl-+2H2O=H2��+Cl2��+2OH-��
1 2
0.025mol n
$\frac{1}{0.025mol}=\frac{2}{n}$����ã�n=0.05mol
C��OH-��=$\frac{0.05mol}{0.5L}$=0.1mol/L��
C��H+��=$\frac{Kw}{C��O{H}^{-}��}$$\frac{1{0}^{-14}}{0.1}$=10-13mol/L��pH=-lg[H+]=13������Ӧ����0.025mol��2=0.05mol��HCl�ָ�ԭ״̬��
�ʴ�Ϊ��13��0.05molHCl��
��5��X�缫����������������Ӧ���缫��ӦʽΪ��2Cu+2OH--2e-=Cu2O+H2O���ʴ�Ϊ��2Cu+2OH--2e-=Cu2O+H2O��
���� ���⿼��ԭ��ء����ع���ԭ������ȷ�ƶ�ȼ�ϵ���������ǽⱾ��Ĺؼ����ѵ�����Һ��pH���㣬���ݵ�ⱥ��ʳ��ˮ�����ӷ���ʽ�������

| A�� | NaCl��HCl | B�� | MgCl2��SO2 | C�� | KCl��CCl4 | D�� | CO2��H2O |
| A�� | v��NH3��=0.010 mol/�� L•s�� | B�� | v��O2��=0.001 mol/�� L•s�� | ||
| C�� | v��NO��=0.001 mol/�� L•s�� | D�� | v��H2O��=0.045 mol/�� L•s�� |
| A�� | 1L 1 mol/L������������������ԼΪ2��6.02��1023�� | |
| B�� | NaHS��Һ��HS-��ˮ�ⷽ��ʽΪ��HS-+H2O?S2-+H3O+ | |
| C�� | A��s��=B��s��+C��g����H=+86 kJ/mol���Է����У�ԭ������ϵ���Է�������Ҷ����ӵķ���ת������� | |
| D�� | ��ˮ�������c��H+��=10-13mol/L����Һ�п��ܺ��У�Fe2+��K+��CO32-��NO3- |
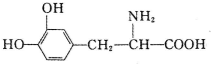
���й���L-������ʵ���������ȷ���ǣ�������
| A�� | �����£�������ˮ | B�� | �ȿ����ᷴӦ���ֿ���Ӧ | ||
| C�� | ��FeCl3��Һ�ޱ仯 | D�� | ����ˮ��ϣ���ˮ��ɫ����Һ������ |
| Ԫ�� | I1 | I2 | I3 | I4 |
| X | 500 | 4 600 | 6 900 | 9 500 |
| Y | 580 | 1 820 | 2 750 | 11 600 |
| A�� | Ԫ��X�ij������ϼ���+1 | |
| B�� | Ԫ��Y�ǵڢ�A��Ԫ�� | |
| C�� | Ԫ��X�����γɻ�����ʱ����ѧʽ������XCl | |
| D�� | ��Ԫ��Y���ڵ�3���ڣ���������ˮ���ҷ�Ӧ |
| A�� |  �ڹ�����������֤�����������ķ�Ӧ �ڹ�����������֤�����������ķ�Ӧ | |
| B�� |  ��ȥ��������������ϩ�ô������� ��ȥ��������������ϩ�ô������� | |
| C�� |  ��ȡ���ռ��������� ��ȡ���ռ��������� | |
| D�� |  ����ʯ�Ͳ��ռ�60��150����� ����ʯ�Ͳ��ռ�60��150����� |
 ����������ԭ��Ӧ��2Ag+��aq��+Cu��s���TCu2+��aq��+2Ag��s����Ƶ�ԭ�����ͼ��ʾ����ش��������⣺
����������ԭ��Ӧ��2Ag+��aq��+Cu��s���TCu2+��aq��+2Ag��s����Ƶ�ԭ�����ͼ��ʾ����ش��������⣺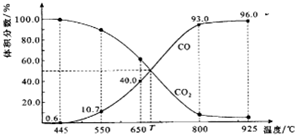 һ������CO2��������̼������ɱ�ĺ�ѹ�ܱ������з�Ӧ��C��s��+CO2��g��?2CO��g����ƽ��ʱ����ϵ����������������¶ȵĹ�ϵ��ͼ��ʾ��
һ������CO2��������̼������ɱ�ĺ�ѹ�ܱ������з�Ӧ��C��s��+CO2��g��?2CO��g����ƽ��ʱ����ϵ����������������¶ȵĹ�ϵ��ͼ��ʾ��