��Ŀ����
����Ŀ�����Ȼ�����(S2C12)�����л�������Ʒ��ɱ�������Ⱦ�ϡ��ϳ��������е��Ȼ������м��塣S2Cl2��һ���ж����ж����ζ��dz��ɫҺ�壬��300��������ȫ�ֽ⣬��������ˮ�ֽ⣬���ܽ���ǣ�����������ͨ�����ڵ���Ƕ��ã�������Cl2�ὫS2Cl2��һ�������õ�SCl2�������Ƕ�S2Cl2���Ʊ����о�����ش��������⣺
��.S2Cl2���Ʊ�
��֪�������ʵ��۷е����£�
���� | S | S2Cl2 | SCl2 |
�е�/�� | 445 | 138 | 59 |
�۵�/�� | 113 | ��76 | ��122 |
�Ʊ�������Ϊ��
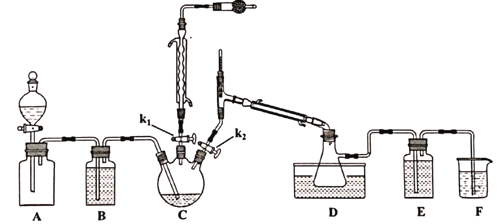
��Aװ�ü���ƿ�г���Cl2����Aװ���еķ�Һ©������k1��k2��һ��ʱ���ر�k2��
�ڽ�ͨ����ˮ����C������135~140�档
��һ��ʱ���ر�k1��ͬʱ��k2�������D���ռ���dz��ɫҺ�塣
(1)ʵ������ȡ�����ķ�Ӧ����ʽ_________________________________��
(2)Aװ�÷�Һ©����ʢװ��Һ����___________��
A.CCl4 B.����NaHCO3��Һ C����NaCl��Һ
(3)�μӹ���Ҫ������ԭ����____________________________________________��
(4)Dװ����ˮԡ�¶�Ӧ����Ϊ___________��ԭ����_______________________________��
��.S2Cl2���ȵIJⶨ
��֪��S2Cl2��ˮ�ֽ�Ļ�ѧ����ʽ��2S2Cl2+2H2O=3S��+SO2��+4HCl��ȡag���л�ɫҺ������ƿ����ˮ����Ӧ��ȫ(�������ʾ�����ˮ��Ӧ)��������Һ�м��������C0 mol/L��AgNO3��ҺV0mL��������ƿ�м����������������ɵij������ǡ���������Fe��NO3)3��ָʾ������C1 mol/L NaSCN��Һ�ζ����յ㣬���� NaSCN��ҺV1mL(�ζ����̷�����Ӧ��Ag++SCN-= AgSCN��)
(5)�ζ��յ������Ϊ_________________________________��
(6)S2Cl2�Ĵ���Ϊ___________(д������ʽ)�����ζ�������δ���������������ô���___________��(�ƫ����ƫС��������Ӱ�족)
���𰸡�Mn02+4HCl![]() MnCl2+Cl2��+2H20 C ����Cl2���٣�ʹCl2��S��ֽӴ�����ֹ����SCl2 ����59��C �������ɵ�S2Cl2��SCl2 ���һ��NaSCN���£���Һ��Ϊdz��ɫ����30s����ȥ
MnCl2+Cl2��+2H20 C ����Cl2���٣�ʹCl2��S��ֽӴ�����ֹ����SCl2 ����59��C �������ɵ�S2Cl2��SCl2 ���һ��NaSCN���£���Һ��Ϊdz��ɫ����30s����ȥ ![]() ƫС
ƫС
��������
I.��1��ʵ������ͨ���ö������̺�Ũ���Ṳ����ȡ������
��2������������ˮ��Ϊ�˽���������ˮ�е��ܽ�ȣ���˳��ʹ������������װ��A��ʢװ��Һ��Ϊ����ʳ��ˮ��
��3���μӹ���Ҫ�����������ٹ��������������������������ὫS2Cl2��һ�������õ�SCl2��
��4��Ϊ��ʹ���ɵ�S2Cl2��SCl2���룬���Ƶ��¶�Ӧ����SCl2�ķе㣻
II. ��5��NaSCN��Һ��Ŀ��Ϊ�ⶨ��Һ��δ���뷴Ӧ�������ӵ����ʵ�����������һ��NaSCN��Һʱ����Һ�е�������ǡ��������ϣ���Fe3++3SCN-= Fe��SCN��3��Fe��SCN��3ΪѪ��ɫ��
��6����������SCN-��Cl-��Ӧ�������ӵ���Դ��S2Cl2��������Ԫ���غ㼴�ɵõ��𰸣�
I.��1��ʵ������ͨ���ö������̺�Ũ���Ṳ����ȡ��������Ӧ����ʽΪ��Mn02+4HCl![]() MnCl2+Cl2��+2H20��
MnCl2+Cl2��+2H20��
��2������������ˮ��Ϊ�˽���������ˮ�е��ܽ�ȣ���˳��ʹ������������װ��A��ʢװ��Һ��Ϊ����ʳ��ˮ����ΪC��
��3���μӹ���Ҫ�����������ٹ��������������������������ὫS2Cl2��һ�������õ�SCl2����Ϊ������Cl2���٣�ʹCl2��S��ֽӴ�����ֹ����SCl2��
��4��Ϊ��ʹ���ɵ�S2Cl2��SCl2���룬���Ƶ��¶�Ӧ����SCl2�ķе㣬��Ϊ������59��C���������ɵ�S2Cl2��SCl2��
II. ��5��NaSCN��Һ��Ŀ��Ϊ�ⶨ��Һ��δ���뷴Ӧ�������ӵ����ʵ�����������һ��NaSCN��Һʱ����Һ�е�������ǡ��������ϣ���Fe3++3SCN-= Fe��SCN��3��Fe��SCN��3ΪѪ��ɫ����Ϊ�����һ��NaSCN���£���Һ��Ϊdz��ɫ����30s����ȥ��
��6����������SCN-��Cl-��Ӧ�������ӵ���Դ��S2Cl2��������Ԫ���غ㼴�ɵõ��𰸣�������n��Ag+��=C0 mol/L��V0mL����NaSCN��Ӧ��n��Ag+��=C1 mol/L��V1mL�����������ӷ�Ӧ��n��Ag+��= C0 mol/L��V0mL- C1 mol/L��V1mL��n��S2Cl2��=![]() n��Cl-��=
n��Cl-��= ![]() ��C0 mol/L��V0mL- C1 mol/L��V1mL������=
��C0 mol/L��V0mL- C1 mol/L��V1mL������=![]() �����ζ�������δ��������������AgCl��s��Ag+��aq��+Cl-��aq����������NaSCN��Һ��Ӧ�����������ʵ���ƫ�ߣ��������ӷ�Ӧ�����������ʵ���ƫ�ͣ�����ƫ�ͣ�
�����ζ�������δ��������������AgCl��s��Ag+��aq��+Cl-��aq����������NaSCN��Һ��Ӧ�����������ʵ���ƫ�ߣ��������ӷ�Ӧ�����������ʵ���ƫ�ͣ�����ƫ�ͣ�

����Ŀ������ʵ�������ȷ���ܴﵽ��Ӧʵ��Ŀ�ĵ���
ѡ�� | ʵ��Ŀ�� | ʵ����� |
A | ����FeCl3��Һ | ��FeCl3�����ܽ�����������ˮ |
B | ��ȡ2.0gNaOH ���� | ���������ϸ���һ����ֽ��Ȼ��������������2g ���룬����������NaOH ���� |
C | ������Һ���Ƿ���NH4+ | ȡ������Һ���Թ��У�����NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ������������� |
D | ��֤����������ʴ | �����������Թ��У��������û |
A. A B. B C. C D. D