��Ŀ����
����Ŀ��ijͬѧ������һƿ��84����Һ������װ˵�����£�

����������Ϣ�����֪ʶ�жϣ����з�������ȷ����
A.�á�84����Һ�������ʵ���Ũ��ԼΪ4.0mol��L-1
B.һƿ�á�84����Һ�������տ�����44.8L��CO2(��״��)����ȫ����
C.ȡ100mL�á�84����Һ��ϡ��100��������������ϡ�ͺ����Һ��c(Na��)ԼΪ0.04mol��L-1
D.���ĸá�84����Һ�����䷽������NaClO��������480mL��25% NaClO������Һ����Ҫ������NaClO��������Ϊ149g
���𰸡�B
��������
A.����Һ�����ʵ���Ũ��c=![]() mol/L=4.0mol/L��A��ȷ��
mol/L=4.0mol/L��A��ȷ��
B.һƿ����84����Һ���к���NaClO�����ʵ���Ϊn(NaClO)=4.0mol/L��1L=4.0mol����������H2CO3>HClO���ᷢ����Ӧ��NaClO+CO2+H2O=NaHCO3+2HClO�����ݷ���ʽ��֪4.0mol NaClO��Ӧ������4mol CO2�����ڱ�״���µ����V=4mol��22.4L/mol=89.6L��B����
C.����84����Һ����Ũ��Ϊ4.0mol/L������ϡ��100����Na+Ũ����ԭ���İٷ�֮һ����ϡ�ͺ����Һ��c(Na��)ԼΪ0.04 mol/L��C��ȷ��
D.û��480mL������ƿ����ʹ��500mL������ƿ��������500mL 25% NaClO������Һ,�����ʵ���Ũ��Ϊ4.0mol/L��n(NaClO)=4.0mol/L��0.5L=2.0mol��m(NaClO)=2.0mol��74.5g/mol=149g��D��ȷ��
�ʺ���ѡ����B��

����Ŀ�����Ȼ�����(S2C12)�����л�������Ʒ��ɱ�������Ⱦ�ϡ��ϳ��������е��Ȼ������м��塣S2Cl2��һ���ж����ж����ζ��dz��ɫҺ�壬��300��������ȫ�ֽ⣬��������ˮ�ֽ⣬���ܽ���ǣ�����������ͨ�����ڵ���Ƕ��ã�������Cl2�ὫS2Cl2��һ�������õ�SCl2�������Ƕ�S2Cl2���Ʊ����о�����ش��������⣺
��.S2Cl2���Ʊ�
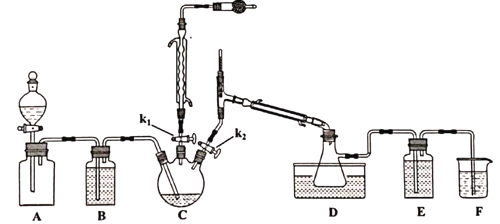
��֪�������ʵ��۷е����£�
���� | S | S2Cl2 | SCl2 |
�е�/�� | 445 | 138 | 59 |
�۵�/�� | 113 | ��76 | ��122 |
�Ʊ�������Ϊ��
��Aװ�ü���ƿ�г���Cl2����Aװ���еķ�Һ©������k1��k2��һ��ʱ���ر�k2��
�ڽ�ͨ����ˮ����C������135~140�档
��һ��ʱ���ر�k1��ͬʱ��k2�������D���ռ���dz��ɫҺ�塣
(1)ʵ������ȡ�����ķ�Ӧ����ʽ_________________________________��
(2)Aװ�÷�Һ©����ʢװ��Һ����___________��
A.CCl4 B.����NaHCO3��Һ C����NaCl��Һ
(3)�μӹ���Ҫ������ԭ����____________________________________________��
(4)Dװ����ˮԡ�¶�Ӧ����Ϊ___________��ԭ����_______________________________��
��.S2Cl2���ȵIJⶨ
��֪��S2Cl2��ˮ�ֽ�Ļ�ѧ����ʽ��2S2Cl2+2H2O=3S��+SO2��+4HCl��ȡag���л�ɫҺ������ƿ����ˮ����Ӧ��ȫ(�������ʾ�����ˮ��Ӧ)��������Һ�м��������C0 mol/L��AgNO3��ҺV0mL��������ƿ�м����������������ɵij������ǡ���������Fe��NO3)3��ָʾ������C1 mol/L NaSCN��Һ�ζ����յ㣬���� NaSCN��ҺV1mL(�ζ����̷�����Ӧ��Ag++SCN-= AgSCN��)
(5)�ζ��յ������Ϊ_________________________________��
(6)S2Cl2�Ĵ���Ϊ___________(д������ʽ)�����ζ�������δ���������������ô���___________��(�ƫ����ƫС��������Ӱ�족)
����Ŀ���ؽ���Ԫ�ظ��Ķ��Խϴ�����ˮ�辭������������ŷš�
��.ij��ҵ��ˮ����Ҫ����Cr3����ͬʱ������������Fe3����Al3����Ca2����Mg2���ȣ������Խ�ǿ��Ϊ�������ã�ͨ�������������̴�����
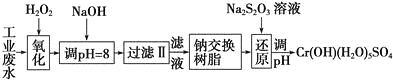
ע�����������ӳ�����������������ʽ��ȫ����ʱ��Һ��pH���±���
�������� | Fe(OH)3 | Fe(OH)2 | Mg(OH)2 | Al(OH)3 | Cr(OH)3 |
pH | 3.7 | 9.6 | 11.1 | 8 | 9(>9�ܽ�) |
��1�����������пɴ���H2O2������Լ���________(�����)��
A��Na2O2B��HNO3C��FeCl3D��KMnO4
��2������NaOH��Һ������ҺpH��8ʱ����ȥ��������________����֪�����ӽ�����֬��ԭ����Mn����nNaR�D��MRn��nNa�����˲�������������ȥ������������__________��
A��Fe3��B��Al3��C��Ca2��D��Mg2��
��3����ԭ�����У�ÿ����0.8 mol Cr2O![]() ת��4.8 mol e�����÷�Ӧ���ӷ���ʽΪ________________��
ת��4.8 mol e�����÷�Ӧ���ӷ���ʽΪ________________��
��.���������£����۸���Ҫ��Cr2O![]() ��ʽ���ڣ���ҵ�ϳ��õ�ⷨ������Cr2O
��ʽ���ڣ���ҵ�ϳ��õ�ⷨ������Cr2O![]() �ķ�ˮ��
�ķ�ˮ��
�÷���Fe���缫��⺬Cr2O![]() �����Է�ˮ�����ŵ����У�������������ҺpH���ߣ�����Cr(OH)3��Һ��
�����Է�ˮ�����ŵ����У�������������ҺpH���ߣ�����Cr(OH)3��Һ��
��1�����ʱ�ܷ���Cu�缫������Fe�缫��________(������������������)��������______________��
��2�����ʱ����������Һ��Cr2O![]() ת��ΪCr3�������ӷ���ʽΪ___________________��
ת��ΪCr3�������ӷ���ʽΪ___________________��
��3�������£�Cr(OH)3���ܶȻ�Ksp��1��10��32����Һ��pHӦΪ____ʱ����ʹc(Cr3��)����10��5mol��L��1��
