��Ŀ����
��֪�������������p��ѹǿ����V���������T���¶ȣ���n�����ʵ�����������������״̬����pV=nRT��ijѧУ����С����Բⶨ��������״̬���������峣��R��ֵ�������Dzⶨʵ��ķ������棬����д�йؿհף�

��һ���ⶨԭ��������������״̬����pV=nRT�У����峣��R=pV/nT����ֵ����ͨ��ʵ����ȷ������ʵ��ͨ������þ�û��������е������ⶨR��ֵ���䷴ӦΪ��
Mg+2HCl=MgCl2+H2��
�����ȡһ��������þ����������ᷴӦ������һ���¶Ⱥ�ѹ���£�����ͨ�������Ӧ�ų������������ʵ���ҵ��¶Ⱥ�ѹ�����Էֱ����¶ȼƺ���ѹ�Ʋ�á��������ʵ�������ͨ����Ӧ��þ����������á����������ø������ݴ���R=" pV" /nTʽ�У��������Rֵ��
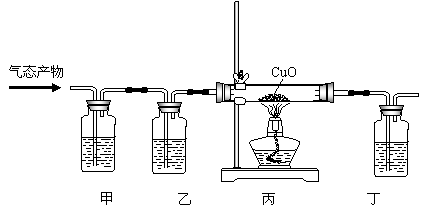
������ʵ����Ʒ���Լ�����������������ƽ���ⶨ���峣����װ�ã�����ͼ��ʾ�������Լ���6mol��L-1HCl��þ�����ɡ�
������ʵ�����ݣ�
1����������ƽ�ϳƳ�þ��������������þ����ƽ������ʾ������ͼ��ʾ��δ�����룬��λΪg����
2������ͼ���������ȡ���Թܣ��ƶ�������B��ʹ������A�е�ˮ���Ե�����̶��ߣ�Ȼ��������B�̶���
3�����Թ��м���15mL 6 mol��L-1HCl����Ҫʹ����մʪ�Թܵ��ϰ벿�����ѳ��ص�þմ����ˮ�������Թ��ϲ���������Ӵ���
4����������Ƿ�©�����������£�������Ҫ�����Ч��
��
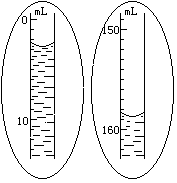
5�����װ�ò�©��������������B��λ�ã�ʹ������A��ˮ����������B ��ˮ����ͬһˮƽ���ϣ�Ҫ����ͬһˮƽ���ϡ���ԭ���� ��ʵ�֡���ͬһˮƽ���ϡ��IJ����� ����Ȼ��ȷ����������A��ˮ�氼����͵�Ķ���V1������ͼ��ʾ����
6������ҡ���Թܣ�ʹþ�����������У�þ�������ᷴӦ�ų���������ʱ������A��ˮ�漴��ʼ�½���Ϊ�˲�ʹ������A����ѹ��������©������������A��ˮ���½���ͬʱ������ ���ϻ��£���������B��ʹ�����ڵ�ˮ���������ˮƽ����Ӧֹͣ���Թ���ȴ�����£�Լ10���ӣ����ƶ�������B��ʹ�����ڵ�ˮ����ƽ��������Ӧ��������A�ڵľ�ȷ����V2������ͼ��ʾ����
7����¼ʵ��ʱ������t�ʹ���ѹP������֪����Ϊ27�棬����ѹΪ100kPa��
���ģ����ݼ�¼�봦�����ں�������д�ʵ����ݣ���
���峣��R��ֵ��������̺ͽ������
���壩���������ۣ����ڶ�ȡ���������������V1ʱ���۾����Ӷ�����������R�IJⶨֵ ��ƫ�ߡ�ƫ�ͻ���Ӱ�죩������û�ȷ�Ӧ�Թ���ȴ�����¾���ȡҺ��ĸ߶ȣ�������R�IJⶨֵ ��ƫ�ߡ�ƫ�ͻ���Ӱ�죩��


��һ���ⶨԭ��������������״̬����pV=nRT�У����峣��R=pV/nT����ֵ����ͨ��ʵ����ȷ������ʵ��ͨ������þ�û��������е������ⶨR��ֵ���䷴ӦΪ��
Mg+2HCl=MgCl2+H2��
�����ȡһ��������þ����������ᷴӦ������һ���¶Ⱥ�ѹ���£�����ͨ�������Ӧ�ų������������ʵ���ҵ��¶Ⱥ�ѹ�����Էֱ����¶ȼƺ���ѹ�Ʋ�á��������ʵ�������ͨ����Ӧ��þ����������á����������ø������ݴ���R=" pV" /nTʽ�У��������Rֵ��
������ʵ����Ʒ���Լ�����������������ƽ���ⶨ���峣����װ�ã�����ͼ��ʾ�������Լ���6mol��L-1HCl��þ�����ɡ�
������ʵ�����ݣ�
1����������ƽ�ϳƳ�þ��������������þ����ƽ������ʾ������ͼ��ʾ��δ�����룬��λΪg����
2������ͼ���������ȡ���Թܣ��ƶ�������B��ʹ������A�е�ˮ���Ե�����̶��ߣ�Ȼ��������B�̶���
3�����Թ��м���15mL 6 mol��L-1HCl����Ҫʹ����մʪ�Թܵ��ϰ벿�����ѳ��ص�þմ����ˮ�������Թ��ϲ���������Ӵ���
4����������Ƿ�©�����������£�������Ҫ�����Ч��
��
5�����װ�ò�©��������������B��λ�ã�ʹ������A��ˮ����������B ��ˮ����ͬһˮƽ���ϣ�Ҫ����ͬһˮƽ���ϡ���ԭ���� ��ʵ�֡���ͬһˮƽ���ϡ��IJ����� ����Ȼ��ȷ����������A��ˮ�氼����͵�Ķ���V1������ͼ��ʾ����
6������ҡ���Թܣ�ʹþ�����������У�þ�������ᷴӦ�ų���������ʱ������A��ˮ�漴��ʼ�½���Ϊ�˲�ʹ������A����ѹ��������©������������A��ˮ���½���ͬʱ������ ���ϻ��£���������B��ʹ�����ڵ�ˮ���������ˮƽ����Ӧֹͣ���Թ���ȴ�����£�Լ10���ӣ����ƶ�������B��ʹ�����ڵ�ˮ����ƽ��������Ӧ��������A�ڵľ�ȷ����V2������ͼ��ʾ����
7����¼ʵ��ʱ������t�ʹ���ѹP������֪����Ϊ27�棬����ѹΪ100kPa��
���ģ����ݼ�¼�봦�����ں�������д�ʵ����ݣ���
| þ�������� | w= | | g |
| ���������ʵ��� | n= | | mol |
| ��Ӧǰ������A�ڶ��� | V1= | | ml |
| ��Ӧ��������A�ڶ��� | V2= | | ml |
| ��������� | V | | ml |
| ���� | T | | K |
| ����ѹ | P | | Pa |
���壩���������ۣ����ڶ�ȡ���������������V1ʱ���۾����Ӷ�����������R�IJⶨֵ ��ƫ�ߡ�ƫ�ͻ���Ӱ�죩������û�ȷ�Ӧ�Թ���ȴ�����¾���ȡҺ��ĸ߶ȣ�������R�IJⶨֵ ��ƫ�ߡ�ƫ�ͻ���Ӱ�죩��
������4������B�ܣ�ʹB����Һ�����A�ܣ�ͨ���۲�A����Һ���Ƿ��½���֪�Ƿ�©������A����Һ�治�½�����˵�������Ժã���A����Һ���½�����˵��װ��©����
5��ʹA��B������Һ����ͬһˮƽ���ϵ�ԭ����ʹ��������ѹǿ��ȣ���Ϊ����ѹ�������ڵķ����������ƶ�B�ܼ��ɡ�
6����
���ģ�
���峣��Rֵ�ļ�����̣�
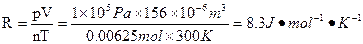
���壩��ƫС ��ƫ��
5��ʹA��B������Һ����ͬһˮƽ���ϵ�ԭ����ʹ��������ѹǿ��ȣ���Ϊ����ѹ�������ڵķ����������ƶ�B�ܼ��ɡ�
6����
���ģ�
| þ�������� | w= | 0.15 | g |
| ���������ʵ��� | n= | 0.00625 | mol |
| ��Ӧǰ������A�ڶ��� | V1= | 2.8 | ml |
| ��Ӧ��������A�ڶ��� | V2= | 158.8 | ml |
| ��������� | V | 156 | ml |
| ���� | T | 300 | K |
| ����ѹ | P | 1��105 | Pa |
���壩��ƫС ��ƫ��
�������Ŀ������ʵ��ⶨ����ѧ�е���������״̬���������峣��R����ֵ��ʵ��ͨ������þ�����ᷴӦ�û����������ⶨR��ֵ����Ȼ��һ������ʵ���ۺ��⡣��ĿҪ��ѧ��������֪ʶ�ͻ�ѧʵ����Ƶ�˼���л���ϣ���������ѧ�е�ѹǿ֪ʶ��ȷ��ȡ�����ܵĶ���ֵ��������ʵ��Ҫ������йصIJ��������ݵĴ�����������ۺ϶Ƚ�ǿ����ѧ��������Ҫ��ϸߡ�������Ū��ʵ��ԭ������ȡһ��������þ����������ᷴӦ�������Ӧ�ų����������V��ʵ���ҵ��¶�T��ѹ��P���Էֱ����¶ȼƺ���ѹ�Ʋ�á��������ʵ���n����ͨ����Ӧ��þ����������á����������ø������ݴ���R=" pV" /nTʽ�У��������Rֵ����������Ƿ�©���ļ���Ч�ķ���������ͨ������B�ܣ�ʹB����ˮ����һ����ѹǿ��ͨ���۲�Һ���Ƿ��½���֪�Ƿ�©������ȡ��������Һ����͵����ֵʱ������ʹA��B������Һ����ͬһˮƽ���ϣ�ֻ����������������������ѹǿ��ȣ���Ϊ����ѹ�������ڵķ����������ƶ�B�ܼ��ɡ������ڶ���ʱ�������뽫�ζ��ܵĶ�������Ǩ���������е������ܡ�0�̶������Ϸ���Ӧ�������¶������ڶ�ȡ���������������V1ʱ���۾����Ӷ��������V1ֵƫ���������������������£����������V2��V1��ֵƫС������R=" pV" /nT��֪��R�IJⶨֵƫ�ͣ���û�ȷ�Ӧ�Թ���ȴ�����¾���ȡҺ��ĸ߶ȣ����V2ֵƫ�����������V2��V1��ֵƫ��R�IJⶨֵƫ�ߡ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
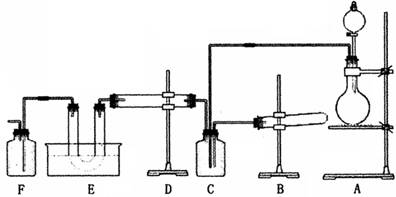

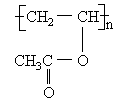
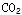 ��CO��
��CO��