��Ŀ����
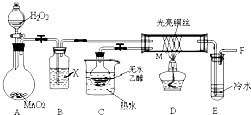
��һ��ijͬѧӦ��������ʾװ���о����ʵ����ʣ���������A����Ҫ�ɷ�������������������������ˮ��������ش��������⣺
��1��Ũ�����������______��
��2��B�й۲쵽��ʵ��������______��
��3�����������ʷ���������������ʵ����ƻ������¹�������Ӧ��δ������뻭��װ��ͼ��������ͼ�ڣ����û�ѧ����ʽ����ԭ��______
���������Ƶ���ˮ���еķ��Ӻ����ӣ��ֱַ�������ʵ�飺
����ɫʯ����Һ���룬��Һ�Ժ�ɫ�������õ�����______��������Һ����ȥ�������õ�����______��
����AgNO3��Һ���в�����ϡ����İ�ɫ�������ɣ������õ�����______��
�������ڱ�״���£�35.5g�����������______L��������������ȫ���ϣ������������ʵ�����______mol�������ɵ������Ƴ�1L��Һ�����ʵ����ʵ���Ũ����______mol?L-1��������Һȡ��20mL������ˮϡ�ͳ�200mL��Һ����ϡϡ�����Һ�����ʵ����ʵ���Ũ����______mol?L-1��

��1��Ũ�����������______��
��2��B�й۲쵽��ʵ��������______��
��3�����������ʷ���������������ʵ����ƻ������¹�������Ӧ��δ������뻭��װ��ͼ��������ͼ�ڣ����û�ѧ����ʽ����ԭ��______
���������Ƶ���ˮ���еķ��Ӻ����ӣ��ֱַ�������ʵ�飺
����ɫʯ����Һ���룬��Һ�Ժ�ɫ�������õ�����______��������Һ����ȥ�������õ�����______��
����AgNO3��Һ���в�����ϡ����İ�ɫ�������ɣ������õ�����______��
�������ڱ�״���£�35.5g�����������______L��������������ȫ���ϣ������������ʵ�����______mol�������ɵ������Ƴ�1L��Һ�����ʵ����ʵ���Ũ����______mol?L-1��������Һȡ��20mL������ˮϡ�ͳ�200mL��Һ����ϡϡ�����Һ�����ʵ����ʵ���Ũ����______mol?L-1��
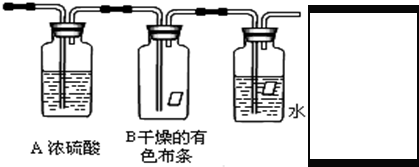
��һ����1��Ũ���������ˮ�ԣ����������������ʵ������������a�е�ˮ������
�ʴ�Ϊ����ȥ�����е�ˮ������
��2����������������Ư���ԣ�װ��B�е���ɫ��������ɫ��
�ʴ�Ϊ������ɫ��
��3�������ж���Ӧ����β�����������������ŷŵ������У����ü���Һ�����գ�����ʽΪCl2+2NaOH=NaCl+NaClO+H2O��װ��ͼΪ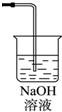 ��
��
�ʴ�Ϊ�� ��Cl2+2NaOH=NaCl+NaClO+H2O��
��Cl2+2NaOH=NaCl+NaClO+H2O��
����������ʹ��ɫʯ����Һ���ɫ����ˮ��Һ��������������ȫ���������ӵ����ᣬ����ʹ��ɫʯ����Һ���ɫ�����������ӣ�
��������Ư���ԣ����Ժ�ɫ��Һ����ɫ�������õ����Ǵ�������ӣ�
�����Ӻ������������ɲ�����ϡ����İ�ɫ���������Լ���AgNO3��Һ���в�����ϡ����İ�ɫ�������ɣ������õ������������ӣ�
�ʴ�Ϊ��H+��HClO��Cl-��
�������ڱ�״���£�35.5g�����������V=n��Vm=
��Vm=
��22.4L/mol=11.2L��
��H2��Cl2��2HCl��֪n��H2��=n��Cl2 ��=
=0.5mol��n��HCl��=2n��Cl2 ��=1mol��
�����ɵ������Ƴ�1L��Һ�����ʵ����ʵ���Ũ����
=1mol/L��
��ϡ�ͺ���Һ��Ũ��ΪC����1mol/L��20mL=C��200mL����ã�C=0.1mol/L��
�ʴ�Ϊ��11.2��0.5��1��0.1��
�ʴ�Ϊ����ȥ�����е�ˮ������
��2����������������Ư���ԣ�װ��B�е���ɫ��������ɫ��
�ʴ�Ϊ������ɫ��
��3�������ж���Ӧ����β�����������������ŷŵ������У����ü���Һ�����գ�����ʽΪCl2+2NaOH=NaCl+NaClO+H2O��װ��ͼΪ
 ��
���ʴ�Ϊ��
 ��Cl2+2NaOH=NaCl+NaClO+H2O��
��Cl2+2NaOH=NaCl+NaClO+H2O������������ʹ��ɫʯ����Һ���ɫ����ˮ��Һ��������������ȫ���������ӵ����ᣬ����ʹ��ɫʯ����Һ���ɫ�����������ӣ�
��������Ư���ԣ����Ժ�ɫ��Һ����ɫ�������õ����Ǵ�������ӣ�
�����Ӻ������������ɲ�����ϡ����İ�ɫ���������Լ���AgNO3��Һ���в�����ϡ����İ�ɫ�������ɣ������õ������������ӣ�
�ʴ�Ϊ��H+��HClO��Cl-��
�������ڱ�״���£�35.5g�����������V=n��Vm=
| m |
| M |
| 35.5g |
| 71g/mol |
��H2��Cl2��2HCl��֪n��H2��=n��Cl2 ��=
| 35.5g |
| 71g/mol |
�����ɵ������Ƴ�1L��Һ�����ʵ����ʵ���Ũ����
| 1mol |
| 1L |
��ϡ�ͺ���Һ��Ũ��ΪC����1mol/L��20mL=C��200mL����ã�C=0.1mol/L��
�ʴ�Ϊ��11.2��0.5��1��0.1��

��ϰ��ϵ�д�
�����Ŀ