��Ŀ����
ij��ѧ����С���������ͼ��ʾ��ʵ��װ�ã����С����Ĵ�������ʵ�飨�̶�װ��ȥ����
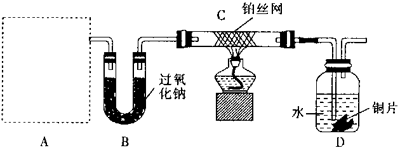
��1��A��ʹ�õ�ҩƷ�ǵ����ʵ�����̼�����ƺ�̼����泥��������������У�A��δ������������ ������ţ���
�ٷ�Һ©�� ���Թ� ����ƿ �ܾƾ��� �ݵ��� ������
��2����װ��B�е�ҩƷ������Ӧ�������� ���ѧʽ����װ��c�з�����������Ӧ�Ļ�ѧ����ʽΪ
��3����A��B��ҩƷ��������װ��D�п��Թ۲쵽�������� ��
��4��ָ����ʦ�Ӱ�ȫ�뻷���Ƕȿ��ǣ�ָ����װ�����������Ե�ȱ�ݣ���������Ľ��飺 ��

��1��A��ʹ�õ�ҩƷ�ǵ����ʵ�����̼�����ƺ�̼����泥��������������У�A��δ������������ ������ţ���
�ٷ�Һ©�� ���Թ� ����ƿ �ܾƾ��� �ݵ��� ������
��2����װ��B�е�ҩƷ������Ӧ�������� ���ѧʽ����װ��c�з�����������Ӧ�Ļ�ѧ����ʽΪ
��3����A��B��ҩƷ��������װ��D�п��Թ۲쵽�������� ��
��4��ָ����ʦ�Ӱ�ȫ�뻷���Ƕȿ��ǣ�ָ����װ�����������Ե�ȱ�ݣ���������Ľ��飺 ��
��1���ڢܢݢޣ���2��CO2��H2O��4NH3��5O2 4NO��6H2O����3��ͭƬ���ܽⲢ�������ݣ���Һ����ɫ�������ڹ��ƿ�Ϸ���Ϊ����ɫ����4����װ��C��D֮������һ��������װ�ã���װ��D��������һ��β������װ�á�
4NO��6H2O����3��ͭƬ���ܽⲢ�������ݣ���Һ����ɫ�������ڹ��ƿ�Ϸ���Ϊ����ɫ����4����װ��C��D֮������һ��������װ�ã���װ��D��������һ��β������װ�á�
 4NO��6H2O����3��ͭƬ���ܽⲢ�������ݣ���Һ����ɫ�������ڹ��ƿ�Ϸ���Ϊ����ɫ����4����װ��C��D֮������һ��������װ�ã���װ��D��������һ��β������װ�á�
4NO��6H2O����3��ͭƬ���ܽⲢ�������ݣ���Һ����ɫ�������ڹ��ƿ�Ϸ���Ϊ����ɫ����4����װ��C��D֮������һ��������װ�ã���װ��D��������һ��β������װ�á���1��A��ʹ�õ�ҩƷ̼�����ƺ�̼������ǹ��壬���ȹ��������������������Ǣ��Թܡ��ܾƾ��ơ��ݵ��ܺ͢���������2���ɣ�1���м���̼�����ƺ�̼����鱗���CO2��H2O��NH3����Na2O2��Ӧ��������CO2��H2O����B�з�Ӧ����O2��NH3����Ӧ��4NH3��5O2 4NO��6H2O����3����A��B��ҩƷ��������C�з�Ӧ���ɵ�NO���������O2��Ӧ����NO2��NO2��D����ˮ��Ӧ����HNO3��HNO3����Cu��Ӧ����ῴ��ͭƬ���ܽⲢ��������NO���壬����Cu2������Һ����ɫ�������ڹ��ƿ�Ϸ�NO��O2��Ӧ����NO2�������Ϊ����ɫ����4��װ��C��D֮������ѹǿ��ԭ��Ӧ��װ��C��D֮������һ��������װ�ã�װ��D�в�����NO2�ж�����Ⱦ������Ӧ��װ��D��������һ��β������װ�á�
4NO��6H2O����3����A��B��ҩƷ��������C�з�Ӧ���ɵ�NO���������O2��Ӧ����NO2��NO2��D����ˮ��Ӧ����HNO3��HNO3����Cu��Ӧ����ῴ��ͭƬ���ܽⲢ��������NO���壬����Cu2������Һ����ɫ�������ڹ��ƿ�Ϸ�NO��O2��Ӧ����NO2�������Ϊ����ɫ����4��װ��C��D֮������ѹǿ��ԭ��Ӧ��װ��C��D֮������һ��������װ�ã�װ��D�в�����NO2�ж�����Ⱦ������Ӧ��װ��D��������һ��β������װ�á�
 4NO��6H2O����3����A��B��ҩƷ��������C�з�Ӧ���ɵ�NO���������O2��Ӧ����NO2��NO2��D����ˮ��Ӧ����HNO3��HNO3����Cu��Ӧ����ῴ��ͭƬ���ܽⲢ��������NO���壬����Cu2������Һ����ɫ�������ڹ��ƿ�Ϸ�NO��O2��Ӧ����NO2�������Ϊ����ɫ����4��װ��C��D֮������ѹǿ��ԭ��Ӧ��װ��C��D֮������һ��������װ�ã�װ��D�в�����NO2�ж�����Ⱦ������Ӧ��װ��D��������һ��β������װ�á�
4NO��6H2O����3����A��B��ҩƷ��������C�з�Ӧ���ɵ�NO���������O2��Ӧ����NO2��NO2��D����ˮ��Ӧ����HNO3��HNO3����Cu��Ӧ����ῴ��ͭƬ���ܽⲢ��������NO���壬����Cu2������Һ����ɫ�������ڹ��ƿ�Ϸ�NO��O2��Ӧ����NO2�������Ϊ����ɫ����4��װ��C��D֮������ѹǿ��ԭ��Ӧ��װ��C��D֮������һ��������װ�ã�װ��D�в�����NO2�ж�����Ⱦ������Ӧ��װ��D��������һ��β������װ�á�
��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ
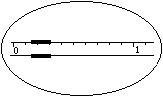
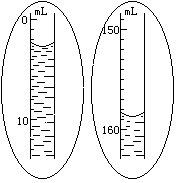
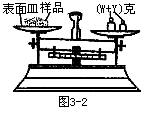 ��1�� ijѧ������֪����y�˵ı�����ȷ��ȡW����Ʒ������������ƽ�����̷��루W+y�������룬�����̵ı������м�����Ʒ����ʱָ��ƫ���ұߣ�����ͼʾ���벹��������IJ�����_______��
��1�� ijѧ������֪����y�˵ı�����ȷ��ȡW����Ʒ������������ƽ�����̷��루W+y�������룬�����̵ı������м�����Ʒ����ʱָ��ƫ���ұߣ�����ͼʾ���벹��������IJ�����_______��