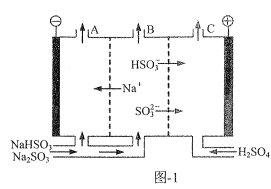
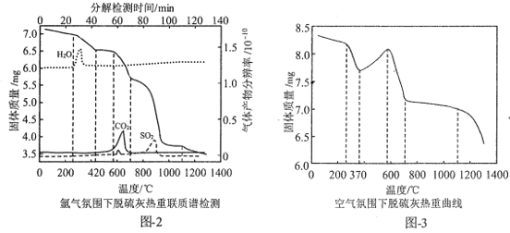
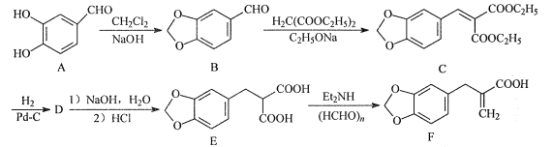
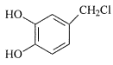
��Ŀ����
����Ŀ��ij��ѧ��ȤС���ͬѧΪ����֤�����Ļ�ѧ��ɣ�����������ʵ�飺
��1����������ˮ��Һ�ֱ�������Թ��У�����ɫ��Ӧ������ɫ�ܲ����۲����Ϊ��ɫ��˵������________________��
��2��������һ���Թ��У����������ˮ������������ɫ��������������Ϊ�����֣��ֱ���������������������ƣ�����������˵��������Һ�к�____________(���ӷ���)��д����ɫ����������������Һ��Ӧ�����ӷ���ʽ________________________________________________��
��3������һ���Թ��У����������ữ�����Ȼ�����Һ��������ɫ������˵��������Һ�к�____________________________ (���ӷ���);
��4������ˮ��Һ��pHֵ________7����ԭ����________________________________ (���ӷ���ʽ);��������������ˮ������ԭ����____________________________________��
���𰸡������� Al3+ Al(OH)3+OH-��AlO2-+ 2H2O ![]() ���� Al3����3H2O
���� Al3����3H2O![]() Al(OH)3��3H�� �����������������ԣ�������ˮ�е�����С����������ʹ֮�������ﵽ��ˮЧ��
Al(OH)3��3H�� �����������������ԣ�������ˮ�е�����С����������ʹ֮�������ﵽ��ˮЧ��
��������
��1������ɫ��Ӧ������ɫ�ܲ����۲����Ϊ��ɫ��˵�����м����ӣ��ʴ�Ϊ�������ӣ�
��2��������һ���Թ��У����������ˮ������������ɫ��������ɫ����Ϊ������������������Ϊ�����֣��ֱ���������������������ƣ�����������˵��������Һ�к�Al3+(���ӷ���)����ɫ����Ϊ����������������������Һ��Ӧ����ƫ�����ƺ�ˮ�����ӷ���ʽAl(OH)3+OH-��AlO2-+ 2H2O���ʴ�Ϊ��Al3+��Al(OH)3+OH-��AlO2-+ 2H2O��
��3�����������ữ�����Ȼ�����Һ��������ɫ����������������ı��������ᱵ��˵��������Һ�к�SO42�����ʴ�Ϊ��SO42����
��4������ˮ��Һ��pHֵС��7����ԭ����Al3��������������ˮ��������ᣬAl3����3H2O![]() Al(OH)3��3H�� (���ӷ���ʽ)����������������ˮ������ԭ���������������������ԣ�������ˮ�е�����С����������ʹ֮�������ﵽ��ˮЧ�����ʴ�Ϊ��С�ڣ�Al3����3H2O
Al(OH)3��3H�� (���ӷ���ʽ)����������������ˮ������ԭ���������������������ԣ�������ˮ�е�����С����������ʹ֮�������ﵽ��ˮЧ�����ʴ�Ϊ��С�ڣ�Al3����3H2O![]() Al(OH)3��3H���������������������ԣ�������ˮ�е�����С����������ʹ֮�������ﵽ��ˮЧ����
Al(OH)3��3H���������������������ԣ�������ˮ�е�����С����������ʹ֮�������ﵽ��ˮЧ����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�