��Ŀ����
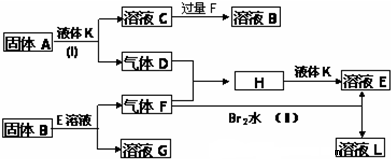
��֪������A����Է�������Ϊ182.5���������ɿ��Ա�ʾΪCxHyOCl���й�ת����ϵ��ͼ��ʾ��
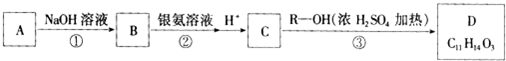 C��һ�������¿ɷ�����Ӧ������һ�ָ߷��ӻ�����E��E�Ľṹ��ʽΪ
C��һ�������¿ɷ�����Ӧ������һ�ָ߷��ӻ�����E��E�Ľṹ��ʽΪ ������R��R��Ϊ��������ش��������⣺
������R��R��Ϊ��������ش��������⣺
��1��A�к��еĺ��������ŵ������� ���۵ķ�Ӧ����Ϊ ��
��2����֪��������R���л���R-OH����50%����A�Ļ�ѧʽΪ ��
��3����֪E��R�������ȡ�����ʶ�λ����E�Ľṹ��ʽΪ ��
��4��д����Ӧ�ٵĻ�ѧ����ʽ ��
��5��D��Ũ���ᡢ���ȵ������·�Ӧ����F��C11H12O2����F��һ�������¿��Է����Ӿ۷�Ӧ��д���üӾ۷�Ӧ�Ļ�ѧ����ʽ ��
��6����B�Ķ���ͬ�������У�д�����ӽṹ�к��� ����������������ͬ���칹��Ľṹ��ʽ ��
����������������ͬ���칹��Ľṹ��ʽ ��
 C��һ�������¿ɷ�����Ӧ������һ�ָ߷��ӻ�����E��E�Ľṹ��ʽΪ
C��һ�������¿ɷ�����Ӧ������һ�ָ߷��ӻ�����E��E�Ľṹ��ʽΪ ������R��R��Ϊ��������ش��������⣺
������R��R��Ϊ��������ش��������⣺��1��A�к��еĺ��������ŵ�������
��2����֪��������R���л���R-OH����50%����A�Ļ�ѧʽΪ
��3����֪E��R�������ȡ�����ʶ�λ����E�Ľṹ��ʽΪ
��4��д����Ӧ�ٵĻ�ѧ����ʽ
��5��D��Ũ���ᡢ���ȵ������·�Ӧ����F��C11H12O2����F��һ�������¿��Է����Ӿ۷�Ӧ��д���üӾ۷�Ӧ�Ļ�ѧ����ʽ
��6����B�Ķ���ͬ�������У�д�����ӽṹ�к���
 ����������������ͬ���칹��Ľṹ��ʽ
����������������ͬ���칹��Ľṹ��ʽ���㣺�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
������������A����Է�������Ϊ182.5���������ɿ��Ա�ʾΪCxHyOCl����12x+y=182.5-16-35.5=131��Cԭ�������Ŀ=
=10��11����A�ķ���ʽӦΪC10H11OCl��B�ܷ���������Ӧ����֪B����-CHO���õ�C��-COOH����C��R-OH�õ�D����������R���л���R-OH����50%����R-OH����Է�������=
=32����RΪ����R-OHΪCH3OH������֪C����ʽΪC10H12O3��C���Եõ��߷��ӻ�����E����E�Ľṹ��ʽ��֪C�к���-COOH��-OH����Aˮ��õ�B���ۺϷ�����֪A�к���-CHO��-OH��A�ı��Ͷ�=
=5������A�к��б��������E��R�������ȡ�����ʶ�λ�����E�Ľṹ��ʽ��֪CΪ ����BΪ
����BΪ ��AΪ
��AΪ ����DΪ
����DΪ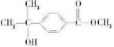 ������л���Ľṹ�������Լ������Ӧ��Ϣ�����⣮
������л���Ľṹ�������Լ������Ӧ��Ϣ�����⣮
| 131 |
| 12 |
| 16 |
| 50% |
| 2��10+2-11-1 |
| 2 |
 ����BΪ
����BΪ ��AΪ
��AΪ ����DΪ
����DΪ ������л���Ľṹ�������Լ������Ӧ��Ϣ�����⣮
������л���Ľṹ�������Լ������Ӧ��Ϣ�����⣮���
�⣺������A����Է�������Ϊ182.5���������ɿ��Ա�ʾΪCxHyOCl����12x+y=182.5-16-35.5=131��Cԭ�������Ŀ=
=10��11����A�ķ���ʽӦΪC10H11OCl��B�ܷ���������Ӧ����֪B����-CHO���õ�C��-COOH����C��R-OH�õ�D����������R���л���R-OH����50%����R-OH����Է�������=
=32����RΪ����R-OHΪCH3OH������֪C����ʽΪC10H12O3��C���Եõ��߷��ӻ�����E����E�Ľṹ��ʽ��֪C�к���-COOH��-OH����Aˮ��õ�B���ۺϷ�����֪A�к���-CHO��-OH��A�ı��Ͷ�=
=5������A�к��б��������E��R�������ȡ�����ʶ�λ�����E�Ľṹ��ʽ��֪CΪ ����BΪ
����BΪ ��AΪ
��AΪ ����DΪ
����DΪ ��
��
��1�������Ϸ�����֪��A���еĺ���������Ϊȩ�����۵ķ�Ӧ����Ϊ������Ӧ��
�ʴ�Ϊ��ȩ����������Ӧ��
��2��������������֪��A�Ļ�ѧʽΪ��C10H11OCl���ʴ�Ϊ��C10H11OCl��
��3��������������֪����E�Ľṹ��ʽΪ��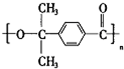 ��
��
�ʴ�Ϊ�� ��
��
��4����Ӧ�ٵĻ�ѧ����ʽΪ��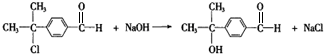 ��
��
�ʴ�Ϊ�� ��
��
��5��DΪ ����Ũ���ᡢ���ȵ������·�Ӧ��ȥ��Ӧ����F��C11H12O2����FΪ
����Ũ���ᡢ���ȵ������·�Ӧ��ȥ��Ӧ����F��C11H12O2����FΪ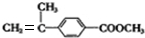 ��F�����Ӿ۷�Ӧ�ķ���ʽΪ��
��F�����Ӿ۷�Ӧ�ķ���ʽΪ��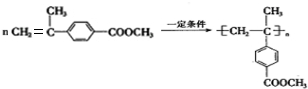 ��
��
�ʴ�Ϊ��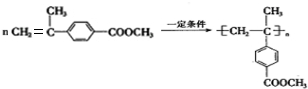 ��
��
��6��BΪ �����ӽṹ�к���
�����ӽṹ�к��� ����������ͬ���칹���У�
����������ͬ���칹���У�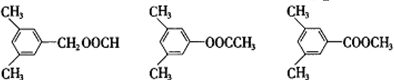 ��
��
�ʴ�Ϊ�� ��
��
| 131 |
| 12 |
| 16 |
| 50% |
| 2��10+2-11-1 |
| 2 |
 ����BΪ
����BΪ ��AΪ
��AΪ ����DΪ
����DΪ ��
����1�������Ϸ�����֪��A���еĺ���������Ϊȩ�����۵ķ�Ӧ����Ϊ������Ӧ��
�ʴ�Ϊ��ȩ����������Ӧ��
��2��������������֪��A�Ļ�ѧʽΪ��C10H11OCl���ʴ�Ϊ��C10H11OCl��
��3��������������֪����E�Ľṹ��ʽΪ��
 ��
���ʴ�Ϊ��
 ��
����4����Ӧ�ٵĻ�ѧ����ʽΪ��
 ��
���ʴ�Ϊ��
 ��
����5��DΪ
 ����Ũ���ᡢ���ȵ������·�Ӧ��ȥ��Ӧ����F��C11H12O2����FΪ
����Ũ���ᡢ���ȵ������·�Ӧ��ȥ��Ӧ����F��C11H12O2����FΪ ��F�����Ӿ۷�Ӧ�ķ���ʽΪ��
��F�����Ӿ۷�Ӧ�ķ���ʽΪ��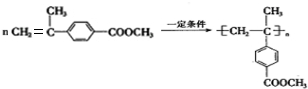 ��
���ʴ�Ϊ��
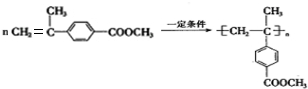 ��
����6��BΪ
 �����ӽṹ�к���
�����ӽṹ�к��� ����������ͬ���칹���У�
����������ͬ���칹���У� ��
���ʴ�Ϊ��
 ��
��
���������⿼���л�����ƶϣ�����ȷ��A�ķ���ʽ�����E�Ľṹ�ص㼰��Ӧ����Ϊͻ�ƿڽ����ƶϣ��ϺõĿ���ѧ���ķ���������������Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
25��ʱ��������Һ��pH���������ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
| A����0.2mol/L��ijһԪ��HA��Һ��0.1mol/L NaOH��Һ�������Ϻ��ҺpH����7����Ӧ��Ļ��Һ��2c��OH-��+c��A-��=2c��H+��+c��HA�� |
| B��pH��Ϊ9��������Һ��CH3COOH��Na2CO3��NaOH�������ʵ���Ũ�ȵĴ�С˳����NaOH ��Һ��CH3COOH��Һ��Na2CO3��Һ |
| C��pH=3�Ķ�Ԫ����H2R��Һ��pH=11��NaOH��Һ��Ϻ��Һ��pH����7����Ӧ��Ļ��Һ��c��R2-��+c��HR-��=c��Na+�� |
| D��0.2mol/L NaHCO3��Һ��0.1mol/L NaOH��Һ�������ϣ�c��H+��=c��OH-��+c��HCO3-��+2c��H2CO3�� |
���й��̻���ʵ�漰������ԭ��Ӧ���ǣ�������
������̿�� ����������̲��� ���������Ũ����ۻ� ��װ��Һ���Լ�ƿ���ò����� �����귢ׯ�� ��ʵ��ʹ�õ�Ũ�����Ի�ɫ��
������̿�� ����������̲��� ���������Ũ����ۻ� ��װ��Һ���Լ�ƿ���ò����� �����귢ׯ�� ��ʵ��ʹ�õ�Ũ�����Ի�ɫ��
| A���٢ۢݢ� | B���ڢ� |
| C���ڢۢܢ� | D���٢ڢۢܢݢ� |
ijѧϰС��Ϊ�о��绯ѧԭ���������ͼװ�ã�����������ȷ���ǣ�������
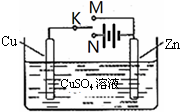

| A��K��M��N���Ͽ�ʱ����װ������Ӧ���� |
| B��K��M��N���Ͽ�ʱ��Zn������Cu���� |
| C��K��M����ʱ��Cu2+��Zn���ƶ� |
| D��K��N����ʱ��Zn�ܽ⣬CuƬ�������� |



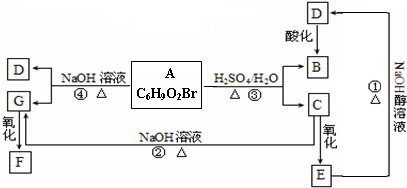
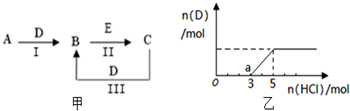 A��B��C��D��EΪ��ѧ��ѧ�����ĵ��ʻ���ת����ϵ��ͼ����ʾ
A��B��C��D��EΪ��ѧ��ѧ�����ĵ��ʻ���ת����ϵ��ͼ����ʾ