��Ŀ����
20��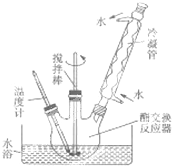 ��������Ǹ�֬���������������֬��״�ͨ��ȡ����Ӧ���������������������´����õ����ò������Ʊ�������͵IJ������£�
��������Ǹ�֬���������������֬��״�ͨ��ȡ����Ӧ���������������������´����õ����ò������Ʊ�������͵IJ������£��ٽ�������ƿ����ƿ�����ﴦ��������������ƿ�м���20g�����ͣ��ٳ�ȡ40g�����飨Լ61mL����
�ڳ�ȡ�״�4.6g��Լ5.8mL���ŵ���ƿ�У�Ȼ���ȡ0.2g�������ƹ��岢ʹ֮�ܽ⣬Ȼ��ӵ�������ƿ�У�
������ͼ��ʾ��װ������ƿ��
�ܺ���ˮԡ���ȣ�ʹ�¶ȱ�����60-65�����ң�����1.5-2h��
��ֹͣ���Ⱥ���ȴ��ȡ��������ƿ�����á���Һ���ϲ�Ϊ�������ͣ�������ͼ״����²���ҪΪ���ͣ�
������ˮϴ���Ƶõ��������3-4�Σ�
�߽�ˮϴ�����Һ����Բ����ƿ�У������¶ȱ�����120�����ң�ֱ����Һ����������ƿ��ʣ���Һ����Ҫ��Ϊ������ͣ�
��1���������Ƶ������Ǵ�����
��2������������������ܼ���
��3��ͼ�������ܵ�����������������
��4������ݷ�Һ���õ�����Ҫ��һ�ֲ��������Ƿ�Һ©����д���ƣ�
��5��ȷ���������ϴ�Ӹɾ��ķ�����ȡ���һ��ϴ��Һ��ˮ���е�Һ�壬��pH��ֽ�ⶨ����pH=7����֤����ϴ�Ӹɾ���
��6����ۣ�1g����������֬���������������صĺ��������IJⶨ
a����ȡ��������Wgע����ƿ�У�����ʯ����-�Ҵ����Һ25mL��ҡ����ƿʹ�����ܽ⣮
b������3�η�̪����0.100mol/L KOH��Һ�ζ���������ɫ�ұ���30s����ʧ������KOH��ҺVmL��
���������͵����Ϊ$\frac{5.6V}{W}$���ú�W��V�Ĵ���ʽ��ʾ��
���� ��1��Ҫ����ˮ�ⷴӦ�У�������������������
��2��������������ˮ������ֱ�����������Ƶȳ�ַֻ�ϣ������������ܼ���ʹ��Ӧ���ܳ�ֻ�ϣ�
��3����Ӧ�м״����л����ӷ���Ϊʹ��Ӧ�ܳ�ֽ��У��Է�Ӧ�����Ҫ����������
��4����Һ�������õ�����Ҫ��һ�ֲ��������Ƿ�Һ©����
��5������ϴ�Ӻ��ϴ��Һ��pHֵ��ȷ��������Ƿ���ϴ�Ӹɾ���
��6����������ɼ������Ӧ�õ����������ص�����Ϊ0.100mol/L��0.001VL��56g/mol=5.6Vmg���������ص�������������͵�����֮�ȼ�Ϊ��������͵���ۣ�
��� �⣺��1��Ҫ����ˮ�ⷴӦ�У�������������������
�ʴ�Ϊ��������
��2��������������ˮ������ֱ�����������Ƶȳ�ַֻ�ϣ������������ܼ���ʹ��Ӧ���ܳ�ֻ�ϣ�
�ʴ�Ϊ�����ܼ���
��3����Ӧ�м״����л����ӷ���Ϊʹ��Ӧ�ܳ�ֽ��У��Է�Ӧ�����Ҫ���������������������ܽ�������������
�ʴ�Ϊ������������
��4����Һ�������õ�����Ҫ��һ�ֲ��������Ƿ�Һ©����
�ʴ�Ϊ����Һ©����
��5������ϴ�Ӻ��ϴ��Һ��pHֵ��ȷ��������Ƿ���ϴ�Ӹɾ�������Ϊȡ���һ��ϴ��Һ��ˮ���е�Һ�壬��pH��ֽ�ⶨ����pH=7����֤����ϴ�Ӹɾ���
�ʴ�Ϊ��ȡ���һ��ϴ��Һ��ˮ���е�Һ�壬��pH��ֽ�ⶨ����pH=7����֤����ϴ�Ӹɾ���
��6����������ɼ������Ӧ�õ����������ص�����Ϊ0.100mol/L��0.001VL��56g/mol=5.6Vmg���������ص�������������͵�����֮�ȼ�Ϊ��������͵���ۣ����Ը�������͵����Ϊ$\frac{5.6V}{W}$��
�ʴ�Ϊ��$\frac{5.6V}{W}$��
���� ���⿼�����л������ķ��뷽����ʵ����̷���Ӧ�ã���Ҫ����Ŀ��Ϣ������Ӧ�ã����ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�
ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�CH3CH2OH$��_{170��}^{H_{2}SO_{4}��Ũ��}$CH2=CH2��CH2=CH2+Br2��BrCH2CH2Br
���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140����ˮ�������ѣ�����������������Ҵ��Ʊ�1��2-���������װ����ͼ��ʾ��
�й������б����£�
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�g/cm3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | -130 | 9 | -116 |
��1���ڴ��Ʊ�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶���ߵ�170�����ң�������ҪĿ����d
a��������Ӧ b���ӿ췴Ӧ�ٶ�c����ֹ�Ҵ��ӷ� d�����ٸ�������������
��2����װ��C��Ӧ����c��������ţ���Ŀ�������շ�Ӧ�п������ɵ���������
a��ˮ b��Ũ����c������������Һ d������̼��������Һ
��3���жϸ��Ʊ���Ӧ�Ѿ�������������������ɫ��ȫ��ȥ
��4����1��2-��������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�����Ӧ���²㣨��ϡ������¡���
��5����������������δ��Ӧ��Br2�������bϴ�ӳ�ȥ������ţ�
a��ˮb������������Һc���⻯����Һd���Ҵ�
��6�������������������������ѣ���������ķ�����ȥ
��7����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ������ϩ���巴Ӧʱ���ȣ���ȴ�ɱ�����Ĵ����ӷ����ֲ��ܹ�����ȴ�����ñ�ˮ������ԭ����1��2-������������̵�ϵͣ�9�棩��������ȴ��ʹ�����̶�ʹ��·������
���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� |
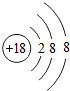 ��
����2���������γ��������������Ԫ����Al����Ԫ�ط��ű�ʾ����д����Ԫ�صĵ����������������ˮ���ﷴӦ�Ļ�ѧ����ʽ2Al+2NaOH+2H2O=2NaAlO2+3H2����
��3 ��������������ˮ�������ʽ��
 �û������������ӣ�����ۡ������ӡ��������
�û������������ӣ�����ۡ������ӡ����������4����������������ˮ�����У�������ǿ����HClO4���ѧʽ��
��5����Ԫ�����Ԫ�����ߺ˵����֮����26��
��6���ڢ����ĵ����У���ѧ���ʽϻ��õ���������������������ʲô��ѧ��Ӧ˵������ʵ��д����Ӧ�����ӷ���ʽ����Cl2+2Br-=2Cl-+Br2��
| A�� | ��״���£�22.4 L H2��CO��������е�ԭ���� | |
| B�� | 1.6 gCH4�к��еĵ����� | |
| C�� | 24 g����þ�����������ᷴӦת�Ƶĵ����� | |
| D�� | 1 mol•L-1��ϡ����100 mL�к���H+����Ŀ |
| A�� | ���з�����Ȼ��й©�������������õ绰���� | |
| B�� | �����ȿ����ھ�ˮ���ֿ�ɱ������ | |
| C�� | ˫��ˮ������ɱ����������Ư�� | |
| D�� | �����Ư�����ܻ��ʹ�ã������ײ����ж����� |