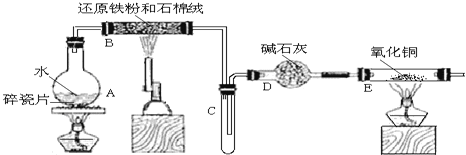
��Ŀ����
����Ŀ������������Ҫ�ɷ���Al2O3����SiO2��Fe2O3�����ʣ�����ȡ�����������ֹ���������ͼ��
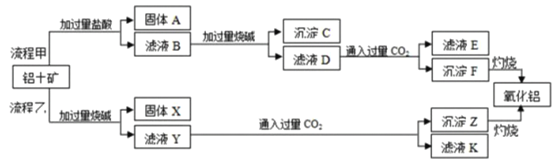
��ش��������⣺
��1������A����Ҫ�ɷ���___��д���ƣ���
��2����������������ᷴӦ�����ӷ���ʽΪ___��___��
��3������������������Ƶõ�������д���÷�Ӧ��ѧ����ʽ____����֪�Ȼ������۵���ͣ���ҵ��Ϊ�β��õ�������Ȼ����ķ����Ʊ��õ���___��
��4���õ��ĵ��������Ը�ǿ����Һ�ų�������ÿת��6.02��1025�����ӣ�ʵ���ϲ��뷴Ӧ��������Ϊ___mol��
���𰸡��������� Al2O3+6H+=2Al3++3H2O Fe2O3+6H+=2Fe3++3H2O 2Al2O3![]() 4Al+3O2�� �Ȼ����Ƿ��Ӿ��壨���ۻ����������״̬�²����� 100
4Al+3O2�� �Ȼ����Ƿ��Ӿ��壨���ۻ����������״̬�²����� 100
��������
���̼ף��������������ϣ��������費��Ӧ���������������������ᷴӦ�����Ȼ������Ȼ��������˺���ҺB���ټӹ����������ƣ��Ȼ������ɳ������Ȼ�������ƫ��������Һ�����ˣ���ҺD�������̼��Ӧ������������������̼��������Һ��
�����ң����������������ƻ�ϣ�����������Ӧ���������跴Ӧ���ɹ����ƣ���������Ӧ����ƫ�����ƣ����ˣ���ҺY�������̼��Ӧ��������������������
�Ŷ������費�����ᷴӦ������A����Ҫ�ɷ��Ƕ������裬�ʴ�Ϊ�������裻
����������������ᷴӦ����Ҫ���������������������ᷴӦ�������ӷ���ʽΪAl2O3+6H+=2Al3++3H2O��Fe2O3+6H+=2Fe3++3H2O���ʴ�ΪAl2O3+6H+=2Al3++3H2O Fe2O3+6H+=2Fe3++3H2O��
�ǵ���������������Ƶõ��������÷�Ӧ�Ļ�ѧ����ʽΪ2Al2O3![]() 4Al+3O2������Ȼ�Ȼ����۵�ͣ������Ƿ��Ӿ���(���ۻ�����)������״̬�²��ܵ��磬���ܵ�����������ʴ�Ϊ2Al2O3
4Al+3O2������Ȼ�Ȼ����۵�ͣ������Ƿ��Ӿ���(���ۻ�����)������״̬�²��ܵ��磬���ܵ�����������ʴ�Ϊ2Al2O3![]() 4Al+3O2�� �Ȼ����Ƿ��Ӿ��壨���ۻ����������״̬�²����룻
4Al+3O2�� �Ȼ����Ƿ��Ӿ��壨���ۻ����������״̬�²����룻
�ȵõ��ĵ��������Ը�ǿ����Һ�ų�������ת��6.02��1025�����Ӽ�ת�Ƶ������ʵ���Ϊ100 mol����������������Һ��Ӧʵ��������ˮ��Ӧ�����������Ƶ������£����ɵ��������������������ܽ⣬ʵ����������ˮ����Ӧ����ʽΪ2Al+2NaOH+6H2O =2NaAlO2+3H2��+4H2O��
2Al+2NaOH+6H2O = 2NaAlO2+3H2��+4H2O ת�Ƶ���
6 mol 6 mol
x 100 mol
![]() =
=![]() �����x = 100 mol�����뷴Ӧ��������Ϊ100 mol���ʴ�Ϊ100 mol��
�����x = 100 mol�����뷴Ӧ��������Ϊ100 mol���ʴ�Ϊ100 mol��

 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�����Ŀ�������Ǽ�����Ҫ�Ļ���ԭ�ϣ��ڹ�ҵ��ũҵ��ҽҩ�����µ�����Ӧ�ù㷺����ҵ��ͨ���ýӴ��������ᣬ��Ҫԭ����������Ϳ������Ӵ�����������������̴��¿ɷ�Ϊ�����Σ������������ȡ�;�������������ת��Ϊ��������������������պ���������ɡ�Ϊ�˷�ֹ������Ⱦ����β�������ۺ����ã����᳧���ð�ˮ����β����SO2��SO3�����壬��������Һ�м���Ũ���ᣬ����ȡ��Ũ�ȵ�SO2����NH4��2SO4��NH4HSO4���塣Ϊ�˲ⶨ������NH4��2SO4��NH4HSO4�����������ɣ��ֳ�ȡ����Ʒ�ķݣ��ֱ������ͬŨ�ȵ�NaOH��Һ50.00mL��������120�����ң�ʹ����ȫ���ݳ�[��NH4��2SO4��NH4HSO4�ķֽ��¶Ⱦ�����200��]������й�ʵ���������£���״������
ʵ�� | ��Ʒ������/g | NaOH��Һ�����/mL | ���������/L����״���� |
1 | 7.24 | 50.00 | 1.792 |
2 | 14.48 | 50.00 | 3.584 |
3 | 21.72 | 50.00 | 4.032 |
4 | 36.20 | 50.00 | 2.240 |
��1����1������ֱ���Ʋ⣺1.81g��Ʒ����ͬ��ʵ��ʱ�����ɰ������������״����Ϊ___L��
��2���Լ���û�����У�NH4��2SO4�� NH4HSO4�����ʵ���֮��Ϊ___��
��3��������NaOH��Һ�����ʵ���Ũ��___mol/L��