��Ŀ����
����Ŀ��CuSO4��Һ����ѧ��ѧ��ũҵ�����г�����һ���Լ���
��1��ijͬѧ����CuSO4��Һʱ�������һ������������Һ�������ӷ���ʽ˵����ԭ����____________��
��2����ͬѧ�����Ƶõ�CuSO4��Һ����������ʵ��̽����
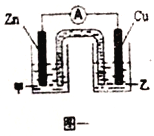
��ͼһ�Ǹ��ݷ�ӦZn+CuSO4=Cu+ZnSO4��Ƴɵ�пͭԭ��ء��������Һ����_______(����ZnSO4������CuSO4��)��Һ;Cu���ĵ�巴Ӧʽ��_______��
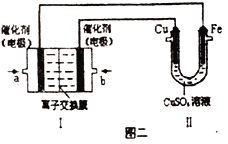
��ͼ���У����Ǽ���ȼ�ϵ��(�������ҺΪKOH��Һ)�Ľṹʾ��ͼ����ͬѧ��������ʵ�����϶�ͭ����a��ͨ�����_______(����CH4������O2��)��a���缫�Ϸ����ĵ缫��Ӧʽ��_____________����ֻ�����е缫����Ϊ���Ե缫�����ʱ�Ļ�ѧ��Ӧ����ʽΪ_____________________���������е缫����Ϊ���Ե缫�����Һ��Ϊ����0.04molCuSO4��0.04molNaCl�Ļ����Һ400mL������������������Ϊ672mL(��״����)ʱ����Һ��pH=_______(���������Һ�������)��
���𰸡� Cu2++2H2O![]() Cu(OH)2+2H+ CuSO4 Cu2++2e-=Cu CH4 CH4-8e-+10OH-=CO32-+7H2O 2CuSO4+2H2O
Cu(OH)2+2H+ CuSO4 Cu2++2e-=Cu CH4 CH4-8e-+10OH-=CO32-+7H2O 2CuSO4+2H2O![]() 2Cu+O2��+2H2SO4 1
2Cu+O2��+2H2SO4 1
����������1������CuSO4��Һʱ�������һ��������������ͭ����ˮ����ͭ����ˮ������ӷ�����Cu2++2H2O![]() Cu(OH)2+2H+��
Cu(OH)2+2H+��
��2����ͼһ�Ǹ��ݷ�ӦZn+CuSO4=Cu+ZnSO4��Ƴɵ�пͭԭ���������пΪ������ͭΪ�������������Һ����CuSO4��Һ;Cu���ĵ�巴Ӧʽ��Cu2++2e-=Cu��
��ͼ���У����Ǽ���ȼ�ϵ��(�������ҺΪKOH��Һ)�Ľṹʾ��ͼ����ͬѧ���ڢ���ʵ�����϶�ͭ������Ϊ�Ƽ��������������Ʋ����ͭ������������a�Ǹ������˴�ͨ�����CH4��a���缫�Ϸ����ĵ缫��Ӧʽ��CH4-8e-+10OH-=CO32-+7H2O����ֻ�Ѣ��е缫����Ϊ���Ե缫�����ʱ�Ļ�ѧ��Ӧ����ʽΪ2CuSO4+2H2O![]() 2Cu+O2��+2H2SO4�����Ѣ��е缫����Ϊ���Ե缫�����Һ��Ϊ����0.04molCuSO4��0.04molNaCl�Ļ����Һ400mL������������������Ϊ672mL(��״����)ʱ�����Լ���������������ʵ���Ϊ0.03mol���������������ӵķŵ�˳����������ǿ����������0.04molCl-��������0.02mol Cl2������һ������0.01mol�������ɣ������Ϲ�ת��0.08mol ������.������0.04molCu2+�ŵ���������0.08mol ���ӡ����Լ�����Һ��pHʱ��ֱ�Ӹ��������������ĵ����������㼴�ɣ��� n(H+)=n(OH-)=4 n(O2)=0.04mol������c(H+)=0.1mol/L����Һ��pH=1��
2Cu+O2��+2H2SO4�����Ѣ��е缫����Ϊ���Ե缫�����Һ��Ϊ����0.04molCuSO4��0.04molNaCl�Ļ����Һ400mL������������������Ϊ672mL(��״����)ʱ�����Լ���������������ʵ���Ϊ0.03mol���������������ӵķŵ�˳����������ǿ����������0.04molCl-��������0.02mol Cl2������һ������0.01mol�������ɣ������Ϲ�ת��0.08mol ������.������0.04molCu2+�ŵ���������0.08mol ���ӡ����Լ�����Һ��pHʱ��ֱ�Ӹ��������������ĵ����������㼴�ɣ��� n(H+)=n(OH-)=4 n(O2)=0.04mol������c(H+)=0.1mol/L����Һ��pH=1��

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�