��Ŀ����
��16�֣����ȫ�����س���������������������Ҫ��Ⱦ���ǿ����������PM2.5�͵������������(PM2.5ֱ���ӽ�2.5��10-6m��1����=10-9m)����Ҫ��ԴΪ��ҵ������������β���ȡ���˶�PM2.5��SO2��NOx�����о������������ش�
(1) ���й���PM2.5˵����ȷ����
a.PM2.5�ڿ������γ��˽��壻
b.PM2.5������������������ж����к����ʣ�
c.�ٿ�˽�ҳ�������ѡ�����������У�ij�̶ֳȿ��Լ���PM2.5��Ⱦ
(2) ȡPM2.5����������ˮ�����Ƶ�����������ø���������ˮ���������Ӽ���ƽ��
Ũ�����±�
| ���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
| Ũ��/ mol/L | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
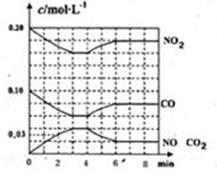
��3��������β���е�NOx�����о�
��NOx���γ����꣬д��NO2ת��ΪHNO3�Ļ�ѧ����ʽ ��
������β��ϵͳ��װ�д�ת�������ɽ�NOx��ԭ��N2�ų�����֪�����Ȼ�ѧ����ʽ��
�� N2(g) +O2(g)
 2NO(g) ��H=+180.5kJ��mol-1
2NO(g) ��H=+180.5kJ��mol-1 �� 2C(s)+ O2(g)
 2CO(g) ��H ="-" 221.0 kJ��mol-1
2CO(g) ��H ="-" 221.0 kJ��mol-1�� C(s)+ O2(g)
 CO2( g) ��H ="-" 393.5 kJ��mol-1
CO2( g) ��H ="-" 393.5 kJ��mol-1�¶����ߣ���Ӧ����ѧƽ�ⳣ�� ���������С�����䡱��
д��NO(g)��CO(g) ��Ӧ����N2(g) CO2(g)���Ȼ�ѧ����ʽ ��
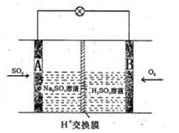
��4�������������в�����SO2ͨ���������̣�����ת��Ϊ��Ӧ�ü�ֵ������Ƶ�
��д����Ӧ��Ļ�ѧ����ʽ�� ��
�������У���Ӧ�����Һ�м���ǿ��ԭ�ԵĶԱ����ӵ����ʣ�Ŀ���� ��
�ۼ��龭����Ӧ��õ��İ�̬������SO42-�����Լ��� ��
��16�֣�
��1��b��c ��2�֣�
(2)���� 4 ����1�֣���2�֣�
��3�� �� 3NO2 + H2O = 2HNO3 + NO ��2�֣�
�ڼ�С ��2�֣�
2NO (g)+2 CO (g) =N2(g)+2CO2(g) ��H ="-" 746.5kJ/mol ��2�֣�
��4���� 2CaCO3 +O2 +2SO2�� 2CaSO4 + 2CO2 ��2�֣�
�ڷ�ֹ�������(NH4) 2 SO3������ ��2�֣�
��������Ȼ�����HCl �� BaCl2) ��2�֣�
���������������1��a��PM2.5��ָ��ֱ����2.5��10-6������������ֱ��Ϊ1��100nm������10-9��10-7��֮�䣬���ڿ����в�ֻ�γ��˽��壬������Һ����a����b��PM2.5ֱ��С��������������������ж����к����ʣ���b��ȷ��c���ٿ�˽�ҳ�������ѡ�����������У�ij�̶ֳȿ��Լ���PM2.5��Ⱦ����c��ȷ����ѡbc��
��2���۲�����з���NH4+ˮ�������ԣ�PM2.5�������Ϊ���ԣ�������pHֵ������Һ�е���غ��c(K+)+c(Na+)+c(NH4+)+c(H+)=2c(SO42-)+c(NO3-)+c(Cl-)����c(H+)= 10-4mol/L��pHֵΪ4��
��3����NO2ת��ΪHNO3�Ļ�ѧ����ʽΪ��3NO2+H2O=2HNO3+NO��
�ڢ�N2��g��+O2��g���T2NO��g����H=+180.5kJ?mol-1 ��
��2C��s��+O2��g���T2CO��g����H=-221.0kJ?mol-1 ��
��C��s��+O2��g���TCO2�� g����H=-393.5kJ?mol-1 ��
��Ӧ������Ӧ���ȣ��¶�����ƽ�������ƶ�����Ӧ��Ũ������������Ũ�ȼ�С����ѧƽ�ⳣ����С��
�ɸ�˹���ɣ�NO��g����CO��g����Ӧ����N2��g����CO2��g�����Ȼ�ѧ����ʽ���ɢۡ�2-��-�ڵõ�����2NO��g��+2CO��g��=N2��g��+2CO2��g����H=-746.5kJ/mol��
��4���ٸ��ݷ�Ӧ��������д����Ӧ��Ļ�ѧ����ʽΪ��2CaCO3+O2+2SO2=2CaSO4+2CO2��
��������������ױ������е�����������������Ӧ�����Һ�м���ǿ��ԭ�ԵĶԱ����ӵ����ʣ�Ŀ���Ƿ�ֹ������泥�NH4��2SO3��������
�ۼ���SO42-�����Լ���������Ȼ�����HCl��BaCl2����
���㣺���鳣�������������Ⱦ����������Ӧ�Ⱥ��ʱ䣻�Ȼ�ѧ����ʽ����ѧƽ�ⳣ���ĺ��壻��������Ļ�ѧ���ʣ���������ӵļ���

 ��У����ϵ�д�
��У����ϵ�д���������Ҫ�ɷ�ΪFeS2���ǹ�ҵ��ȡ�������Ҫԭ�ϣ���ȼ�ղ���ΪSO2��Fe2O3��
��1����֪1g FeS2��ȫȼ�շų�7.1kJ���������ʾFeS2��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽΪ��
______________________________________________________________��
��2����0.050molSO2(g)��0.030molO2(g)�����ݻ�Ϊ1L���ܱ������У���Ӧ��2SO2(g)��O2(g)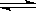 2SO3(g) ��һ�������´ﵽƽ�⣬���c(SO3)=0.040mol/L����������·�Ӧ��ƽ�ⳣ��K����ֵΪ___________,SO2��ƽ��ת����Ϊ__________��
2SO3(g) ��һ�������´ﵽƽ�⣬���c(SO3)=0.040mol/L����������·�Ӧ��ƽ�ⳣ��K����ֵΪ___________,SO2��ƽ��ת����Ϊ__________��
��3�����÷�Ӧ����ƽ��״̬ʱ����ʹƽ��������Ӧ�����ƶ��ҷ�Ӧ���ʼӿ죬���д�ʩ���е��� ��������ĸ��
| A����ƽ�������г���Ar | B����ƽ�������г���O2 |
| C���ı䷴Ӧ�Ĵ��� | D�����ͷ�Ӧ���¶� |
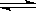 2SO3(g) ��H<0 �� SO2��ת��������ʼ�¶�T1=673K���淴Ӧʱ�䣨t���ı仯����ͼ�������������䣬���ı���ʼ�¶�ΪT2=723K������ͼ�л����¶�T2��SO2��ת�����淴Ӧʱ��仯��Ԥ�ڽ��ʾ��ͼ��
2SO3(g) ��H<0 �� SO2��ת��������ʼ�¶�T1=673K���淴Ӧʱ�䣨t���ı仯����ͼ�������������䣬���ı���ʼ�¶�ΪT2=723K������ͼ�л����¶�T2��SO2��ת�����淴Ӧʱ��仯��Ԥ�ڽ��ʾ��ͼ��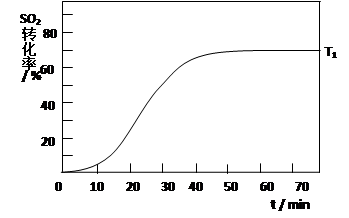
��7�֣�(1)���������ھ�������������ˮ������������ҵ�������ΪƯ����
�ٳ������������������ҿ������������е��ʷ�Ӧ���磺
6Ag(s)+O3(g)===3Ag2O(s)����H =" -235.8" kJ/mol;
��֪��2 Ag2O(s)===4Ag(s)+O2(g)����H = +62.2kJ/mol;
��Ӧ 2O3��g��= 3O2��g�� �ġ�H = kJ/mol��
�ڿ�ѧ��P��Tatapudi��������ʹ�������������µ��ˮ�ķ����Ƶó�����������������Χ��ˮ�в�
�����������������������ɹ������⣬�����缫��ӦʽΪ �� ��2���û���̿��ԭ��������������йط�ӦΪ��C(s)+2NO(g) N2(g)+CO2(g) ij�о�С����ij�ܱյ��������(��������������䣬��������������Բ���)�м���NO�������Ļ���̿������(T1��) �����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
��2���û���̿��ԭ��������������йط�ӦΪ��C(s)+2NO(g) N2(g)+CO2(g) ij�о�С����ij�ܱյ��������(��������������䣬��������������Բ���)�м���NO�������Ļ���̿������(T1��) �����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�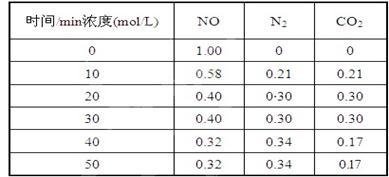
����10 min��20 min��ʱ����ڣ���CO2��ʾ�ķ�Ӧ����Ϊ ��
��д���÷�Ӧ��ƽ�ⳣ���ı���ʽK= ��
�����и�������Ϊ�жϸ÷�Ӧ�ﵽƽ��״̬���� (�������ĸ)��
| A��������ѹǿ���ֲ��� | B��2v��(NO)=v��(N2) |
| C��������CO2������������� | D�����������ܶȱ��ֲ��� |
��һ���¶��£�����NO����ʼŨ��������NO��ƽ��ת���� (����������䡱��С��)��
�����ߡ�������д���ҹ�������ҵ����ƪ�¡����������Ҫ���ܵ�ȼ�ϣ�ͨ������(N2H4)��Ϊȼ�ϣ�N2O4����������
��1����֪��N2(g) + 2O2(g) =2NO2(g) ��H����67.7 kJ��mol��1
N2H4(g) + O2(g) =N2(g) + 2H2O(g) ��H����534.0 kJ��mol��1
2NO2(g) N2O4(g) ��H����52.7 kJ��mol��1
N2O4(g) ��H����52.7 kJ��mol��1
��д����̬������̬������������ȼ�����ɵ�������̬ˮ���Ȼ�ѧ����ʽ�� ��
��2����ҵ�Ͽ��ô�������������İ���Ӧ�Ʊ��£��÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
��3��һ�������£���2L�ܱ���������ʼͶ��2 mol NH3��4 mol O2������Ӧ��
4NH3(g)+5O2(g�� 4NO(g)+6H2O(g) ��H<0
4NO(g)+6H2O(g) ��H<0
���ƽ��ʱ�������£�
| ƽ��ʱ���ʵ�����mol�� | n(NO) | n(H2O�� |
| �¶�T1 | 1.6 | 2.4 |
| �¶�T2 | 1.2 | 1.8 |
�����¶�T1�£�������10min��Ӧ�ﵽƽ�⣬��10min�ڷ�Ӧ��ƽ������
v(NH3)�� ��
���¶�T1��T2�Ĵ�С��ϵ��T1 T2���>���� ��<����������
��4�������˺���������̬ϵͳ�У�����Ҫ�����ȥ��CO2����Ҫ���ṩ�����O2��ij�ֵ绯ѧװ�ÿ�ʵ������ת����2CO2=2CO+O2��CO������ȼ�ϡ���֪�÷�Ӧ��������ӦΪ��
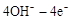 =O2��+2H2O����������ӦΪ �����������������Ʒ�Ӧ2CO=2C+O2����H��0��������CO����Ⱦ�������ж�������Ӧ�Ƿ����Է����в�˵������ ��
=O2��+2H2O����������ӦΪ �����������������Ʒ�Ӧ2CO=2C+O2����H��0��������CO����Ⱦ�������ж�������Ӧ�Ƿ����Է����в�˵������ ����5����ͼ��ij�ռ�վ����ת��ϵͳ�ľֲ�ʾ��ͼ������ȼ�ϵ�ز���KOH��ҺΪ���Һ��
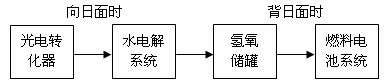
���ij��ʱ�������������й��ռ���33.6L���壨������ɱ�״��������ö�ʱ����ˮ���ϵͳ��ת�Ƶ��ӵ����ʵ���Ϊ mol��
��һ�������£������ܱ������н��еĿ��淴ӦA��g����3B��g��
|
C����λʱ������ n mol A��ͬʱ���� 3nmol B D�� A��B��C�ķ�����֮��Ϊ 1��3��2
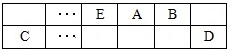
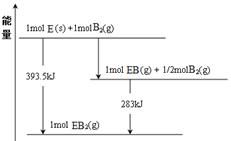

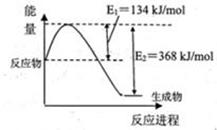
 N2(g)��2CO2(g)�ġ�H�� ��
N2(g)��2CO2(g)�ġ�H�� ��