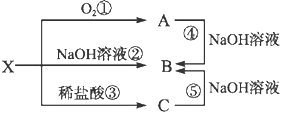
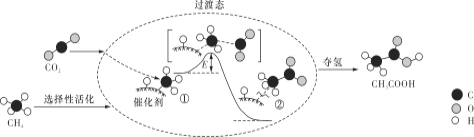
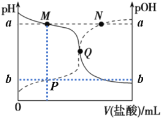
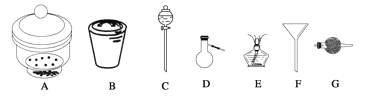
��Ŀ����
����Ŀ������β�������е�һ����Ӧ���£�2NO(g)+2CO(g)N2(g)+2CO2(g)����ش��������⣺
(1)��֪��N2(g)+O2(g)=2NO(g)��H=+180.5kJ��mol-l
C(s)+O2(g)=CO2(g) ��H=-393.5kJ��mol-l
2C(s)+O2(g)=2CO(g) ��H=-221kJ��mol-l
��2NO(g)+2CO(g)N2(g)+2CO2(g)����H=____kJ��mol-l��
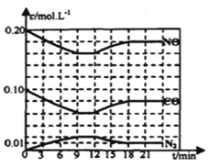
(2)һ���¶��£������Ϊ 1 L���ܱ������г���һ������NO��CO����t1ʱ�̴ﵽƽ��״̬����ʱn(CO)=0.1 mol��n(NO)=0.2 mol��n(N2)= a mol����N2ռƽ���������![]() ��
��
����÷�Ӧ��ƽ�ⳣ��K=______���������¶ȼ�����������䣬ƽ����ڴ˻��������������г���3a mol��N2��0.2 mol��NO��ƽ�⽫______�ƶ�(����������������������������)��
�����и����������˵���÷�Ӧ�Ѿ��ﵽƽ�����______��
A��v����(CO2)= v����(CO)
B�����������ܶȲ��ٸı�
C����������ƽ����Է����������ٸı�
D��NO��CO��N2��CO2��Ũ�Ⱦ����ٱ仯
E����λʱ��������2n mol̼��˫����ͬʱ����n molN��N
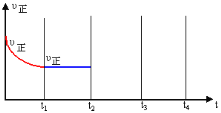
����t2ʱ�̣�������Ѹ��ѹ����ԭ�ݻ���![]() ���������������������£� t3ʱ�̴ﵽ�µ�ƽ��״̬������ͼ�в��仭��t2��t3��t4ʱ�Σ�����Ӧ���ʵı仯����_________��
���������������������£� t3ʱ�̴ﵽ�µ�ƽ��״̬������ͼ�в��仭��t2��t3��t4ʱ�Σ�����Ӧ���ʵı仯����_________��
(3)Ϊ��������β���е��к�����Դ�������Ⱦ����������װβ������װ�á�����װ����װ�к�Pd�ȹ���Ԫ�صĴ����������ڴ�������������������õĻ�������ͼ��ʾ��д�������仯�е��ܻ�ѧ��Ӧ����ʽ_________��

���𰸡���H=-746.5kJ/mol K��270 �� CD  2NO��O2��4CO
2NO��O2��4CO![]() 4CO2��N2
4CO2��N2
��������
(1)�����Ȼ�ѧ����ʽ��˹���ɼ���õ������Ȼ�ѧ����ʽ��
��N2(g)+O2(g)�T2NO(g)��H=+180.5kJmol-l
��C(s)+O2(g)�TCO2(g)��H=-393.5kJmol-l
��2C(s)+O2(g)�T2CO(g)��H=-221kJmol-l
��˹���ɼ������2-��-�ٵõ��Ȼ�ѧ����ʽ��
(2)�ٸ�������ʽ��ϻ�ѧƽ����ƶ�֪ʶ�����㣻
�ڸ��ݻ�ѧƽ��״̬��������𣬵���Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȣ������ʵ�Ũ�ȡ��ٷֺ������䣬�Լ��ɴ�������һЩ��Ҳ�������仯������ʱҪע�⣬ѡ���жϵ������������ŷ�Ӧ�Ľ��з����仯�������������ɱ仯����ֵʱ��˵�����淴Ӧ����ƽ��״̬��
��ѹ�����������������ѹǿ�����淴Ӧ��������ƽ�������������С�ķ����ƶ����ݴ˻�����t2-t3-t4ʱ�Σ�����Ӧ���ʵı仯���ߣ�
(3)������֪��Ϣ��д��Ӧ��Ͳ��ﲢ��ƽ����ʽ��
(1)��N2(g)+O2(g)�T2NO(g)��H=+180.5kJmol-l
��C(s)+O2(g)�TCO2(g)��H=-393.5kJmol-l
��2C(s)+O2(g)�T2CO(g)��H=-221kJmol-l
��˹���ɼ������2-��-�ٵõ��Ȼ�ѧ����ʽΪ��2NO(g)+2CO(g)N2(g)+2CO2(g)��H=2��(-393.5kJmol-l)-(-221kJmol-l)-(+180.5kJmol-l)=-746.5kJ/mol��
(2)�ٻ�ѧƽ�����м�����ʽ���㣬��t1ʱ�̴ﵽƽ��״̬����ʱn(CO)=0.1mol��n(NO)=0.2mol��n(N2)=a mol����N2ռƽ���������![]() ��������ʽ��
��������ʽ��

��![]() =
=![]() �����a=0.3��K=
�����a=0.3��K= ��ƽ����ڴ˻��������������г���3amol��0.9mol��N2��0.2mol��NO�������ʵ����ֱ�����0.4��0.1��1.2��0.6����ʱQc=
��ƽ����ڴ˻��������������г���3amol��0.9mol��N2��0.2mol��NO�������ʵ����ֱ�����0.4��0.1��1.2��0.6����ʱQc=![]() =270=K�����Բ��ƶ���
=270=K�����Բ��ƶ���
��A��v����(CO2)=v����(CO)������֤�����淴Ӧ������ȣ���A���������⣻
B�����������ܶ�=![]() ����Ӧǰ�������غ㣬���仯��������䣬�����ܶ�ʼ�ղ��䣬���ܶȲ��仯��״̬��һ��ƽ�⣬��B���������⣻
����Ӧǰ�������غ㣬���仯��������䣬�����ܶ�ʼ�ղ��䣬���ܶȲ��仯��״̬��һ��ƽ�⣬��B���������⣻
C����������ƽ����Է�������=![]() �������غ㣬����n�仯������������ƽ����Է����������ٸı䣬�ﵽ��ƽ��״̬����C�������⣻
�������غ㣬����n�仯������������ƽ����Է����������ٸı䣬�ﵽ��ƽ��״̬����C�������⣻
D��NO��CO��N2��CO2��Ũ�Ⱦ����ٱ仯�ǻ�ѧƽ�����������D�������⣻
E����λʱ��������2n mol̼��˫����ͬʱ����nmolN��N������֤�����淴Ӧ������ȣ���E���������⣻
��ѡCD��
��ѹ�����������������ѹǿ�����淴Ӧ��������ƽ�������������С�ķ����ƶ��������������ƶ�������Ӧ���ʴ����淴Ӧ���ʣ�����
 ��
��
(3)NO2Ϊ�м�����Ӧ��ΪNO��O2��CO������ΪCO2��N2����Ӧ����ʽΪ2NO��O2��4CO![]() 4CO2��N2��
4CO2��N2��

����Ŀ����ˮ���Ȼ���������ýȾ�����л��ϳ��е��Ȼ�������ʵ���ҿ������ڵ���![]() �۵�
�۵�![]() ��
��![]() ��Ӧ�Ʊ�
��Ӧ�Ʊ�![]() ��װ����ͼ��
��װ����ͼ��
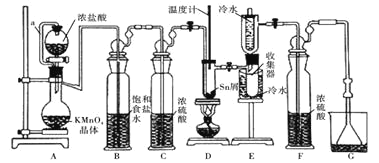
��֪��![]() ��
��![]() �й��������ʣ�
�й��������ʣ�
���� | ��ɫ��״̬ | �۵�/�� | �е�/�� |
SnCl2 | ��ɫ���� | 246 | 652 |
SnCl4 | ��ɫҺ�� | -33 | 114 |
![]() ����ˮ������
����ˮ������![]() ���ش��������⣺
���ش��������⣺
��1������a��������____________��װ��A�з�����Ӧ�����ӷ���ʽΪ____________________��
��2�����۲쵽װ��FҺ���Ϸ�___________ʱ�ſ�ʼ��ȼD���ľƾ��ƣ������ۻ����ʵ����������������������ȡ���ʱ�������ȵ�Ŀ����![]() ________��
________��![]() ______
______
��3��������װ����ȱ��װ��![]() ��������ͬ
��������ͬ![]() ����D����֧�Թ��з�������Ҫ����Ӧ��ѧ����ʽΪ_________________________________��
����D����֧�Թ��з�������Ҫ����Ӧ��ѧ����ʽΪ_________________________________��
��4��![]() �����ķ�Ӧ������
�����ķ�Ӧ��������
![]() ��Ϊ��ֹ��Ʒ�д�������
��Ϊ��ֹ��Ʒ�д�������![]() ���ɲ�ȡ�Ĵ�ʩ��____________________________________________��
���ɲ�ȡ�Ĵ�ʩ��____________________________________________��
��5���ζ�������Ʒ��2��![]() ��
��![]() �ĺ������÷�����ƽ��ȡ
�ĺ������÷�����ƽ��ȡ![]() ��Ʒ����ƿ�У�������ˮ�ܽ⣬���������Һ����
��Ʒ����ƿ�У�������ˮ�ܽ⣬���������Һ����![]() �ĵ����Һ�ζ����յ�ʱ����
�ĵ����Һ�ζ����յ�ʱ����![]() �����Ʒ��
�����Ʒ��![]() ��
��![]() ����������Ϊ___________��
����������Ϊ___________��![]() С�������2λ����֪
С�������2λ����֪![]()