��Ŀ����
17���������ӷ���ʽ����д��ȷ���ǣ�������| A�� | NaAlO2��Һ����NaHCO3�У�H++AlO2-+H2O=Al��OH��3�� | |
| B�� | ��FeI2��Һ�еμ�������ˮ��2Fe2++Cl2=2Fe3++2Cl- | |
| C�� | ��2.24g����Ͷ��400mL 0.3 mol/L��ϡHNO3�У�4Fe+12H++3NO3-=Fe3++3Fe2++3NO��+6 H2O | |
| D�� | ��0.5 mol��������Һ����μ���0.9 mol Ba��OH��2��5Al3++9SO42-+9Ba2++15OH-=9BaSO4��+5Al��OH��3�� |
���� A��̼��������Ӳ��ܲ���Ҫ����������ʽ��
B����ˮ�����������ӵĻ�ԭ�Դ����������ӣ����������ȷ�Ӧ��
C������������������ʵ�����Ȼ�����Fe+4H++NO3-=Fe3++NO��+2H2O��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O�жϹ����������Ӧ�������ʽ��������������������ӵ����ʵ��������ݼ�����д����Ӧ�����ӷ���ʽ��
D���ȼ���������ӡ���������ӡ������Ӻ����������ӵ����ʵ�����Ȼ���жϹ����������Ӧ���T�ɣ�
��� �⣺A��NaAlO2��Һ����NaHCO3�У�����̼��������ӵ����Է�Ӧ������������Ӧ����̼���ƺ�����������������Ӧ�����ӷ���ʽΪ��HCO3-+H2O+AlO2-�TAl��OH��3��+CO32-����A����
B����FeI2��Һ�еμ�������ˮ�����������ȱ���������ȷ�����ӷ���ʽΪ��2I-+Cl2=2I-+2Cl-����B����
C��2.24g���۵����ʵ���Ϊ��$\frac{2.24g}{56g/mol}$=0.04mol��400mL 0.3 mol/L��ϡHNO3�к�����������ʵ���Ϊ��0.3mol/L��0.4L=0.12mol��������������ʵ���֮��Ϊ1��3������Fe+4H++NO3-=Fe3++NO��+2H2O��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O��֪������Ӧ���������������Ӻ������ӣ�������ȫ��Ӧ��������������Ϊxmol��������������Ϊymol����x+y=0.04��4x+$\frac{8}{3}$y=0.12����ã�x=0.01��y=0.03������Ӧ���ɵ����������������ӵ����ʵ���֮��Ϊ1��3���÷�Ӧ�����ӷ���ʽΪ��4Fe+12H++3NO3-=Fe3++3Fe2++3NO��+6H2O����C��ȷ��
D��0.5mol�������к���1mol��������ӡ�0.5mol�����ӣ�0.9 mol Ba��OH��2�к���0.9mol�����Ӻ�1.8mol���������ӣ�����������ӹ�������������ȫ��Ӧ�������ᱵ������0.5mol��������ȫת��������������������1.5mol���������ӣ�ʣ���0.3mol�����������ܹ��ܽ�0.3mol������������Ӧ����0.3molƫ��������Ӻ�0.2mol����������������ȷ�����ӷ���ʽΪ��5Al3++9SO42-+9Ba2++18OH-=3BaSO4��+6H2O+2Al��OH��3��+3AlO2-����D����
��ѡC��
���� ���⿼�������ӷ���ʽ���жϣ������Ǹ߿��еĸ�Ƶ�⣬�����е��Ѷȵ����⣬ע���������ӷ���ʽ����дԭ����ȷ���ӷ���ʽ�����жϳ��÷�����������ؿ��鷴Ӧ�����������������Ӱ�죬C��DΪ�ѵ㡢�״��㣬ע����ȷ�жϷ�Ӧ�������������

| A�� | 2F2+2H2O�T4HF+O2 | |
| B�� | 2CH3COOH+Ca��ClO��2�T2HClO+Ca��CH3COO��2 | |
| C�� | I2+2NaClO3�T2NaIO3+Cl2 | |
| D�� | 4HCl+MnO2�TMnCl2+Cl2��+2H2O |
| A�� | ��ȥ������̼�л��е�����һ����̼���壬ͨ�������������ȼ | |
| B�� | ��ȥ̼�����ƹ�����������̼���ƹ��壬�ü��ȷ� | |
| C�� | ��ȥCO2��������HCl��������ͨ��ʢ�����ռ���Һ��ϴ��ƿ | |
| D�� | ��ȥ���л��е���������������Ͷ������NaOH��Һ����ַ�Ӧ����� |
| A�� | ���� | B�� | С�� | C�� | ���� | D�� | ����ȷ�� |
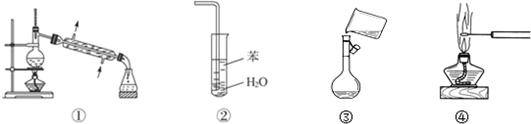
| A�� | װ�âٳ����ڷ��뻥�����ܵ�Һ������ | |
| B�� | װ�âڿ���������NH3������ֹ���� | |
| C�� | ͼ���ǽ��ܽ�õ���Һת�Ƶ�����ƿ�� | |
| D�� | ͼ�ܿɹ۲�NaCl����ɫ��Ӧ |
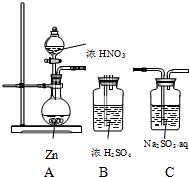 ��ͬѧ̽��NO2�������ԣ����������ʵ��װ�ã�
��ͬѧ̽��NO2�������ԣ����������ʵ��װ�ã���1����װ�õ�����ȱ���ǣ���β������װ��
��2��ʵ�鿪ʼ����Zn�ܽ⣬��δ�����������ݳ���ͬѧ�����������ΪHNO3����ԭ����NH4+д����Ӧ�����ӷ���ʽ��4Zn+NO3-+10H+=4Zn2++NH4++3H2O �����֤�ò��룺ȡ����A�з�Ӧ�����Һ�����Թ��У������еμ���������������Һ�������ȣ����Թܿڴ�����һ��ʪ��ĺ�ɫʯ����ֽ������ֽ��������������
��3�����µ���HNO3Ũ�Ⱥ���A���к���ɫ�����ݳ������ʵ�飬֤��NO2���������ԣ�ҩƷ��ѡ�������ɣ�
| ���� | ���� | ���� |
| ȡ����C�з�Ӧ�����Һ �����Թ��У� �ȼ���������Һ�ữ���ټ��� �Ȼ�����Һ | �а�ɫ�������� | NO2���������� |
��һ��NO2������SO32-
�������HNO3������SO32-
����1��������ǣ����ǡ���������
���ɣ�3NO2+H2O=2HNO3+NO���û�ѧ����ʽ�ش�
����2����Ҫ��֤NO2���������ԣ����ʵ�鷽���ǣ���һ������ܱ�������ͬʱͨ��NO2��SO2��һ��ʱ�������������ĺ���ɫ��ȥ��֤���˶��߷����˷�Ӧ��֤����NO2�������ԣ�
| A�� | ���ռ���Һ�еμ������Ȼ�����Һ��Al3++4OH-�TAlO2-+2H2O | |
| B�� | ���ʵ�����ȵ��廯������������Ӧ��2Fe2++2Br-+2Cl2�T2Fe3++Br2+4Cl- | |
| C�� | �ؾ�������ˮ��S2-+2H2O?2OH-+H2S | |
| D�� | ��̼������Һ�еμӹ�����ϡ���CO32-+2H+�TCO2��+H2O |
| A�� | �ŵ�ʱ������ӦΪ��Zn-2e-+2OH-=Zn��OH��2 | |
| B�� | �ŵ�ʱÿת��3mol���ӣ�������1molK2FeO4������ | |
| C�� | ���ʱ������ӦΪ��Fe��OH��3-3e-+5OH-=FeO42-+4H2O | |
| D�� | �ŵ�ʱ����������Һ�ļ�����ǿ |
