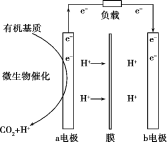
МвДҝДЪИЭ
ЎҫМвДҝЎҝДі°аН¬С§УГТФПВКөСйМҪҫҝ![]() Ўў
Ўў![]() өДРФЦКЎЈ»ШҙрПВБРОКМвЈә
өДРФЦКЎЈ»ШҙрПВБРОКМвЈә
(1)·ЦұрИЎТ»¶ЁБҝВИ»ҜМъЎўВИ»ҜСЗМъЎўде»ҜСЗМъ№ММеЈ¬ҫщЕдЦЖіЙ![]() өДИЬТәЎЈФЪ
өДИЬТәЎЈФЪ![]() ИЬТәЦРРијУИлЙЩБҝМъРјЈ¬ЖдДҝөДКЗ_____________________________________ЎЈ
ИЬТәЦРРијУИлЙЩБҝМъРјЈ¬ЖдДҝөДКЗ_____________________________________ЎЈ
(2)јЧЧйН¬С§ИЎ![]() ИЬТәЈ¬јУИлјёөОВИЛ®Ј¬ФЩјУИл1өО
ИЬТәЈ¬јУИлјёөОВИЛ®Ј¬ФЩјУИл1өО![]() ИЬТәЈ¬ИЬТәұдәмЈ¬ЛөГч
ИЬТәЈ¬ИЬТәұдәмЈ¬ЛөГч![]() ҝЙҪ«
ҝЙҪ«![]() Сх»ҜЎЈ
Сх»ҜЎЈ![]() ИЬТәУлВИЛ®·ҙУҰөДАлЧУ·ҪіМКҪОӘ____________________ЎЈ
ИЬТәУлВИЛ®·ҙУҰөДАлЧУ·ҪіМКҪОӘ____________________ЎЈ
(3)ТТЧйН¬С§ИПОӘјЧЧйөДКөСйІ»№»СПҪчЈ¬ёГЧйН¬С§ФЪ![]() ИЬТәЦРПИјУИл
ИЬТәЦРПИјУИл![]() ГәУНЈ¬ФЩПтПВІгИЬТәЦРТАҙОјУИлјёөОВИЛ®әН1өО
ГәУНЈ¬ФЩПтПВІгИЬТәЦРТАҙОјУИлјёөОВИЛ®әН1өО![]() ИЬТәЈ¬ИЬТәұдәмЎЈГәУНөДЧчУГКЗ_________________________ЎЈ
ИЬТәЈ¬ИЬТәұдәмЎЈГәУНөДЧчУГКЗ_________________________ЎЈ
(4)ұыЧйН¬С§ИЎ![]() ЙПКц
ЙПКц![]() ИЬТәЈ¬ПтЖдЦРөОјУЙЩБҝРВЦЖөДВИЛ®Ј¬ХсөҙәуИЬТәіК»ЖЙ«ЎЈДіН¬С§¶ФІъЙъ»ЖЙ«өДФӯТтМбіцБЛјЩЙиЈә
ИЬТәЈ¬ПтЖдЦРөОјУЙЩБҝРВЦЖөДВИЛ®Ј¬ХсөҙәуИЬТәіК»ЖЙ«ЎЈДіН¬С§¶ФІъЙъ»ЖЙ«өДФӯТтМбіцБЛјЩЙиЈә
јЩЙи1Јә![]() ұ»
ұ»![]() Сх»ҜіЙ
Сх»ҜіЙ![]() ИЬҪвФЪИЬТәЦРЈ»
ИЬҪвФЪИЬТәЦРЈ»
јЩЙи2Јә![]() ұ»
ұ»![]() Сх»ҜіЙ
Сх»ҜіЙ![]() ЎЈ
ЎЈ
ЗлДгНкіЙПВұнЈ¬СйЦӨјЩЙи
КөСйІҪЦиЎўФӨЖЪПЦПу | ҪбВЫ |
ўЩПтИЬТәЦРјУИл____________Ј¬ХсөҙЎўҫІЦГПЦПуЈә____________ | јЩЙи1ХэИ· |
ўЪПтИЬТәЦРјУИл____________Ј¬ПЦПуЈә____________ | јЩЙи2ХэИ· |
(5)ТСЦӘЈә![]() ЎЈИфФЪ
ЎЈИфФЪ![]() ЙПКц
ЙПКц![]() ИЬТәЦРНЁИлұкЧјЧҙҝц
ИЬТәЦРНЁИлұкЧјЧҙҝц![]() Ј¬·ҙУҰөДАлЧУ·ҪіМКҪОӘ________________________________ЎЈ
Ј¬·ҙУҰөДАлЧУ·ҪіМКҪОӘ________________________________ЎЈ
Ўҫҙр°ёЎҝ·АЦ№![]() ұ»Сх»Ҝ
ұ»Сх»Ҝ ![]() ёфАлҝХЖшЈЁЕЕіэСхЖш¶ФКөСйөДУ°ПмЈ©
ёфАлҝХЖшЈЁЕЕіэСхЖш¶ФКөСйөДУ°ПмЈ© ![]() ПВІгіКіИәмЙ«Ј¬ЙПІгіКОЮЙ«[»тұҪЙПІгіКіИәмЙ«Ј¬ПВІгіКОЮЙ«]
ПВІгіКіИәмЙ«Ј¬ЙПІгіКОЮЙ«[»тұҪЙПІгіКіИәмЙ«Ј¬ПВІгіКОЮЙ«] ![]() ИЬТә ИЬТәұдОӘәмЙ«
ИЬТә ИЬТәұдОӘәмЙ« ![]()
ЎҫҪвОцЎҝ
ёщҫЭСЗМъАлЧУөД»№ФӯРФј°МъАлЧУөДјмСй·Ҫ·Ё·ЦОцҪвҙрЈ»ёщҫЭАлЧУ»№ФӯРФЗҝИх·ЦОцСх»ҜөДПИәуЛіРтЈ¬¶ЁБҝјЖЛгКөјКІОјУ·ҙУҰөДАлЧУЈ¬УГАлЧУ·ҪіМКҪұнКҫЎЈ
ЈЁ1Ј©Fe2+ҫЯУР»№ФӯРФЈ¬ТЧұ»ҝХЖшЦРСхЖшСх»ҜұдЦКЈ¬МъәНВИ»ҜМъ·ҙУҰЙъіЙВИ»ҜСЗМъЈ¬ФЪFeCl2ИЬТәЦРРијУИлЙЩБҝМъРјЈ¬ЖдДҝөДКЗ·АЦ№ВИ»ҜСЗМъұ»Сх»ҜЈ¬№Кҙр°ёОӘЈә·АЦ№![]() ұ»Сх»ҜЈ»
ұ»Сх»ҜЈ»
ЈЁ2Ј©ВИЖшҫЯУРСх»ҜРФДЬСх»ҜВИ»ҜСЗМъОӘВИ»ҜМъЈ¬·ҙУҰөДАлЧУ·ҪіМКҪОӘЈә![]() Ј¬№Кҙр°ёОӘЈә
Ј¬№Кҙр°ёОӘЈә![]() Ј»
Ј»
ЈЁ3Ј©ГәУНДСИЬУЪЛ®Ј¬ГЬ¶ИұИЛ®РЎЈ¬·ЦІгәуҝЙТФёфАлИЬТәУлҝХЖшҪУҙҘЈ¬ЕЕіэСхЖш¶ФКөСйөДУ°ПмЈ¬№Кҙр°ёОӘЈәёфАлҝХЖш(ЕЕіэСхЖш¶ФКөСйөДУ°Пм)Ј»
ЈЁ4Ј©ўЩИфОӘјЩЙи1ФтУРBr2ЙъіЙЈ¬ПтИЬТәЦРјУИлCCl4ід·ЦХсөҙЎўҫІЦГЈ¬CCl4ІгПФіИәмЛөГчЙъіЙBr2Ј¬ЛөГчјЩЙи1ХэИ·Ј»ўЪИфОӘјЩЙи2ФтУРFe3+ЙъіЙЈ¬ПтИЬТәјУИлKSCNИЬТәЈ¬ИЬТәұдәмЈ¬ЛөГчУРМъАлЧУЙъіЙЈ¬јЩЙи2ХэИ·Ј¬№Кҙр°ёОӘЈә![]() Ј»ПВІгіКіИәмЙ«Ј¬ЙПІгіКОЮЙ«[»тұҪЙПІгіКіИәмЙ«Ј¬ПВІгіКОЮЙ«] Ј»
Ј»ПВІгіКіИәмЙ«Ј¬ЙПІгіКОЮЙ«[»тұҪЙПІгіКіИәмЙ«Ј¬ПВІгіКОЮЙ«] Ј»![]() ИЬТәЈ»ИЬТәұдОӘәмЙ«Ј»
ИЬТәЈ»ИЬТәұдОӘәмЙ«Ј»
ЈЁ5Ј©ТСЦӘЈә![]() Ј¬ФтВИЖшПИәНСЗМъАлЧУ·ҙУҰЈ¬ФЩәНдеАлЧУ·ҙУҰЈ»n(Cl2)=
Ј¬ФтВИЖшПИәНСЗМъАлЧУ·ҙУҰЈ¬ФЩәНдеАлЧУ·ҙУҰЈ»n(Cl2)=![]() Ј¬n(FeBr2)=0.1mol/LЎБ0.05L=0.005molЈ¬ёщҫЭөГК§өзЧУКШәг·ЦОцөГ·ҙУҰЦРУР0.005molСЗМъАлЧУұ»Сх»ҜЈ¬УР0.005molдеАлЧУұ»Сх»ҜЈ¬ФтАлЧУ·ҪіМКҪОӘЈә
Ј¬n(FeBr2)=0.1mol/LЎБ0.05L=0.005molЈ¬ёщҫЭөГК§өзЧУКШәг·ЦОцөГ·ҙУҰЦРУР0.005molСЗМъАлЧУұ»Сх»ҜЈ¬УР0.005molдеАлЧУұ»Сх»ҜЈ¬ФтАлЧУ·ҪіМКҪОӘЈә ![]() Ј¬№Кҙр°ёОӘЈә
Ј¬№Кҙр°ёОӘЈә![]() ЎЈ
ЎЈ

ЎҫМвДҝЎҝДіН¬С§УГВЛЦҪХЫіЙТ»ёцЦҪәыөыЈ¬ІўЕзИчТ»ЦЦИЬТәЈЁұЈіЦКӘИуЈ©Ј¬№ТФЪМъјЬМЁЙПЈ¬Ҫ«КўДіЦЦИЬТәөДЙХұӯ·ЕФЪЦҪәыөыөДПВ·ҪЈ¬ИзНјЛщКҫЎЈПтЙХұӯЦРјУИлБнТ»ЦЦОпЦКЈ¬№эТ»»б¶щ·ўПЦЦҪәыөыұдОӘәмЙ«ЗТұЈіЦәмЙ«І»ұдЈ¬ПВГжөДЧйәПДЬ№»КөПЦЙПКцұд»ҜөДКЗЈЁ Ј©
A | B | C | D | |
ЦҪәыөыЙПөДЕзИчТә | КҜИп | ·УМӘ | ·УМӘ | КҜИп |
ЙХұӯЦРөДИЬТә | ЕЁБтЛб | ВИ»Ҝп§ | ПЎБтЛб | ёЯГМЛбјШ |
јУИлЙХұӯЦРөДОпЦК | Нӯ | ЗвСх»ҜДЖЕЁИЬТә | °ұЛ® | ЕЁСОЛб |
A.AB.BC.CD.D
ЎҫМвДҝЎҝөВ№ъ»ҜС§јТ№юІ®(F. Haber, 1868-1930)·ўГчөДәПіЙ°ұјјКхК№ҙуЖшЦРөДөӘЖшұдіЙБЛЙъІъөӘ·КөДУАІ»ҝЭҪЯөДБ®јЫАҙФҙЈ¬ҙУ¶шК№Е©ТөЙъІъТААөНБИАөДіМ¶ИјхИхЈ¬ҪвҫцБЛөШЗтЙПТтБёКіІ»ЧгөјЦВөДјў¶цәНЛАНцОКМвЎЈТтҙЛХвО»ҪвҫИКАҪзБёКіОЈ»ъөД»ҜС§МмІЕ»сөГБЛ1918ДкЕөұҙ¶ы»ҜС§ҪұЎЈПЦФЪОТГЗФЪКөСйКТДЈДв№ӨТөЦЖ°ұөД№эіМЈ¬ТФМҪҫҝНвҪзМхјю¶ФЖҪәвөДУ°ПмЎЈ
ІйФДЧКБПЈ¬»сөГТФПВјьДЬКэҫЭЈә
»ҜС§јь | NЎФN | H-H | N-H |
јьДЬ/(kJ/mol) | 946 | 436 | 391 |
(1)јЖЛг№ӨТөәПіЙ°ұ·ҙУҰөД·ҙУҰИИЈәN2(g)+3H2(g)2NH3(g)ЎчH=________kJ/mol
(2)Т»¶ЁОВ¶ИПВЈ¬ПтТ»ёцәгС№ИЭЖчЦРідИлN20.6molЈ¬H20.5molЈ¬ФЪТ»¶ЁОВ¶ИПВҪшРР·ҙУҰЈәN2(g)+3H2(g) 2NH3(g)Ј¬ҙпөҪЖҪәвКұЈ¬N2өДЧӘ»ҜВКОӘ![]() Ј¬ҙЛКұИЭЖчөДМе»эОӘ1LЎЈ
Ј¬ҙЛКұИЭЖчөДМе»эОӘ1LЎЈ
ёГОВ¶ИКұИЭЖчЦРЖҪәвМеПөөДЖҪәвіЈКэКЗ______________ЎЈ
(3)әПіЙ°ұ№ӨТө»бІъЙъҙуБҝёұІъОпCO2Ј¬№ӨТөЙПіЈУГёЯЕЁ¶ИөДK2CO3ИЬТәОьКХCO2Ј¬өГИЬТәXЈ¬ФЩАыУГөзҪв·ЁK2CO3ИЬТәФЩЙъЈ¬ЖдЧ°ЦГИзНјЛщКҫЈә
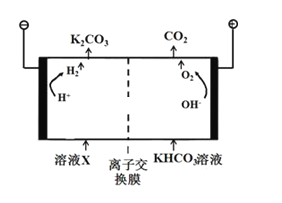
ўЩФЪСфј«Зш·ўЙъөД·ҙУҰ°ьАЁ____________________әНH+Ј«HCO3- ЁTH2OЈ«CO2ЎьЎЈ
ўЪјтКцCO32-ФЪТхј«ЗшФЩЙъөДФӯАн__________ЎЈ
ўЫФЩЙъЧ°ЦГЦРІъЙъөДCO2әНH2ФЪТ»¶ЁМхјюПВ·ҙУҰЙъіЙјЧҙјЈ¬№ӨТөЙПАыУГёГ·ҙУҰәПіЙјЧҙјЎЈ
ТСЦӘЈә25 ЎжЈ¬101 KPaПВЈә
2H2(g)Ј« O2(g)ЁT2H2O(g) ҰӨ H1ЁTЈӯ484kJ/mol
2CH3OH(g)Ј« 3O2(g)ЁT2CO2(g)Ј«4H2O(g) ҰӨ H2ЁTЈӯ1352kJ/mol
РҙіцCO2әНH2ЙъіЙCH3OH(g)әНH2O(g)өДИИ»ҜС§·ҪіМКҪ__________ЎЈ