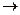��Ŀ����
�����13�֣�����������ѧʵ����ijС���ͬѧ�Ժ�ˮ�л�ѧ��Դ��̽�����

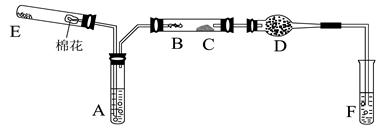
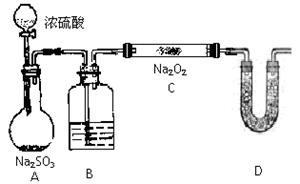
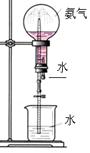
��һ���Ӻ���������ȡ��
�Ӻ���������ȡ���ʵ����������:
(1) ʵ�������У�������٢ڢ۱�ʾ���Dz�������������д��
�� �� ��
(2) ʵ�������У������2~3���ӡ���Ҫ������ ��
��3��ʵ�������У����μ�ϡ����ͼ���H2O2����Ҫ������______________��
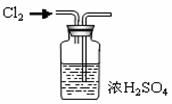
(��) �ⶨ±ˮ������þ�ĺ���
�ú�ˮ���Σ������ʳ�κ��±ˮ����Ҫ�����Ȼ��ƺ�����þ���ⶨ±ˮ������þ������ʵ�鲽�����£�
����ȡ��±ˮ��Ʒ100mL��
��ȡ��һ�����ʵ���Ũ�ȵ�����������Һ100mL��ƽ�����Ĵ����μ��뵽��100mL±ˮ��Ʒ�У������
��ÿ��ʵ���������ݼ��±�
��ش�
��4����д��ʵ����������漰���ķ�Ӧ���ӷ���ʽ____________________��
��5���ϱ���X����Ϊʵ�������©������ֵ�����Ʋ�X����ֵΪ_____��
��6��ʵ�������õ�������������Һ���ʵ���Ũ��Ϊ_______________��
��7��100mL±ˮ�к�����þ��������_________g

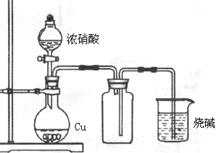
��һ���Ӻ���������ȡ��
�Ӻ���������ȡ���ʵ����������:
(1) ʵ�������У�������٢ڢ۱�ʾ���Dz�������������д��
�� �� ��
(2) ʵ�������У������2~3���ӡ���Ҫ������ ��
��3��ʵ�������У����μ�ϡ����ͼ���H2O2����Ҫ������______________��
(��) �ⶨ±ˮ������þ�ĺ���
�ú�ˮ���Σ������ʳ�κ��±ˮ����Ҫ�����Ȼ��ƺ�����þ���ⶨ±ˮ������þ������ʵ�鲽�����£�
����ȡ��±ˮ��Ʒ100mL��
��ȡ��һ�����ʵ���Ũ�ȵ�����������Һ100mL��ƽ�����Ĵ����μ��뵽��100mL±ˮ��Ʒ�У������
��ÿ��ʵ���������ݼ��±�
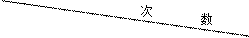 | 1 | 2 | 3 | 4 |
| ��������������Һ�����/mL | 25 | 25 | 25 | 25 |
| ���ɳ�����������/g | 0.29 | X | 0.87 | 0.87 |
��4����д��ʵ����������漰���ķ�Ӧ���ӷ���ʽ____________________��
��5���ϱ���X����Ϊʵ�������©������ֵ�����Ʋ�X����ֵΪ_____��
��6��ʵ�������õ�������������Һ���ʵ���Ũ��Ϊ_______________��
��7��100mL±ˮ�к�����þ��������_________g
�����13�֣�
(1)���ˣ�1�֣� ��ȡ��1�֣� ��Һ��1�֣�
(2) ��ʹ�������к������ʳ���ܽ⣨1�֣�
(3) ����ҺA��I������ΪI2��1�֣�
(4) 2OH��+ Mg+ = Mg(OH)2��2�֣�
(5) 0.58 ��2�֣�
(6) 0.4mol/L ��2�֣�
(7) 1.8��2�֣�
(1)���ˣ�1�֣� ��ȡ��1�֣� ��Һ��1�֣�
(2) ��ʹ�������к������ʳ���ܽ⣨1�֣�
(3) ����ҺA��I������ΪI2��1�֣�
(4) 2OH��+ Mg+ = Mg(OH)2��2�֣�
(5) 0.58 ��2�֣�
(6) 0.4mol/L ��2�֣�
(7) 1.8��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 ��������
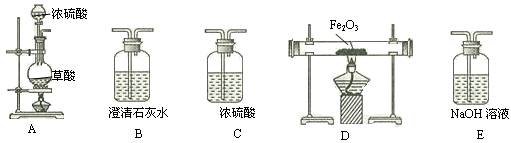
��������
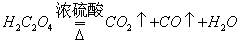 ��
��