��Ŀ����
��10�֣���ͼ��ʾΪij��ѧ��ȤС����Ƶ��Ҵ������������������ʵ��װ��(ͼ�м�������������̨�����еȾ�δ����)��
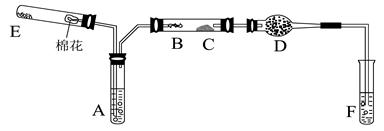
ͼ�У�AΪ��ˮ�Ҵ�(�е�Ϊ78��)��BΪ�Ƴ�����״��ϸͭ˿����˿��CΪ��ˮCuSO4��ĩ��DΪ��ʯ�ң�FΪ���Ƶļ���Cu(OH)2����Һ��
(1)������װ���У�ʵ��ʱ��Ҫ���ȵ�����Ϊ(��������ij��λ�Ĵ���) ��
(2)ΪʹA���Ҵ�ƽ���������Ҵ�����,�����õķ�����_____________��
D��ʹ�ü�ʯ�ҵ������� ��
(3)�����Ҵ���������ʱF�е�ʵ�������� ��
(4) E����һ�ִ�����䷴Ӧ����ʽΪ ��
(5)д���Ҵ������������Ļ�ѧ����ʽ ��

ͼ�У�AΪ��ˮ�Ҵ�(�е�Ϊ78��)��BΪ�Ƴ�����״��ϸͭ˿����˿��CΪ��ˮCuSO4��ĩ��DΪ��ʯ�ң�FΪ���Ƶļ���Cu(OH)2����Һ��
(1)������װ���У�ʵ��ʱ��Ҫ���ȵ�����Ϊ(��������ij��λ�Ĵ���) ��
(2)ΪʹA���Ҵ�ƽ���������Ҵ�����,�����õķ�����_____________��
D��ʹ�ü�ʯ�ҵ������� ��
(3)�����Ҵ���������ʱF�е�ʵ�������� ��
(4) E����һ�ִ�����䷴Ӧ����ʽΪ ��
(5)д���Ҵ������������Ļ�ѧ����ʽ ��
.��10��
��1��E��A��B��F��©ѡ��1�֣���ѡ����ѡ���÷֣� ����2�֣�
��2��ˮԡ���� ��1�֣�
��ֹF�е�ˮ��������C������ˮCuSO4���ã�Ӱ�����ˮ�ļ��顣��2�֣�
��3��F�в�����ɫ��������1�֣�
��4��2KMnO4=K2MnO4+MnO2+O2������2�֣�
��5��2CH3CH2OH+O2��2CH3CHO+2H2O��2�֣�
��1��E��A��B��F��©ѡ��1�֣���ѡ����ѡ���÷֣� ����2�֣�
��2��ˮԡ���� ��1�֣�
��ֹF�е�ˮ��������C������ˮCuSO4���ã�Ӱ�����ˮ�ļ��顣��2�֣�
��3��F�в�����ɫ��������1�֣�
��4��2KMnO4=K2MnO4+MnO2+O2������2�֣�
��5��2CH3CH2OH+O2��2CH3CHO+2H2O��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
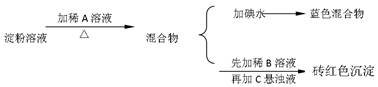
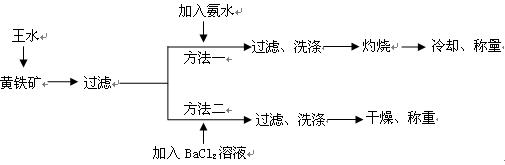
 ��(��=1.42g��cm-3)��3�����Ũ����(��=1.19g��cm-3)��϶��ɵġ�
��(��=1.42g��cm-3)��3�����Ũ����(��=1.19g��cm-3)��϶��ɵġ�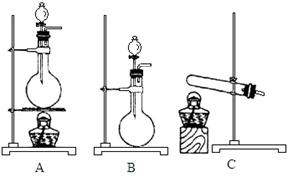
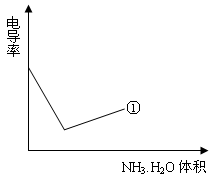
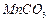 ���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������
���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������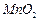 Ϊԭ���Ʊ������ߴ�
Ϊԭ���Ʊ������ߴ� ��Һ��
��Һ��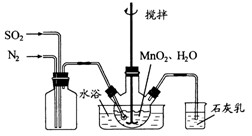
 ��
�� ������壬��Ӧ3h��ֹͣͨ��
������壬��Ӧ3h��ֹͣͨ��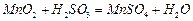 ����
����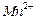 ��
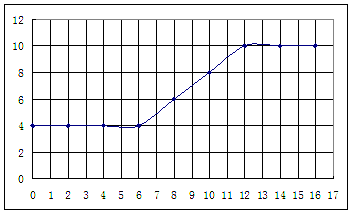
��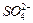 ��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��
��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��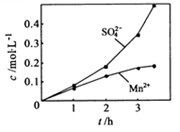
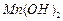 ��ʼ����ʱ
��ʼ����ʱ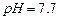 ���벹���ɣ�1���Ƶõ�
���벹���ɣ�1���Ƶõ�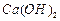 ��
��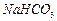 ��
��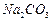 ��
��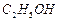 ]��
]��