��Ŀ����
10����ȲΪԭ���ڲ�ͬ�����¿��Ժϳɶ����л��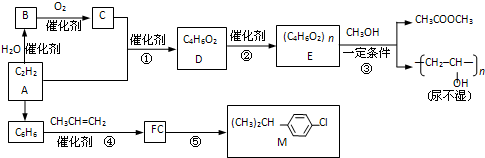
��֪����CH2=CH?OH�����ȶ���$\stackrel{�Զ�}{��}$ CH3CHO
��һ�������£��������ᷢ��������Ӧ��RCOOR��+R��OH$\stackrel{һ������}{��}$ RCOOR��+R��OH
���������գ�
��1��д��Ӧ���ͣ���ȡ����Ӧ��Ӧ���ܼӳɷ�Ӧ��Ӧ����Ӧ�ݵķ�Ӧ����������������
��2��д����Ӧ����ʽ��B����C2CH3CHO+O2$��_{��}^{����}$2CH3COOH����Ӧ��nCH3COOCH=CH2$\stackrel{����}{��}$
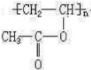 ��
����3��R��M��ͬϵ��仯ѧʽΪ
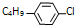 ����R��4�֣�
����R��4�֣���4��д����̼̼˫�����ܷ���������Ӧ����������D��ͬ���칹��Ľṹ��ʽHCOOCH2CH=CH2��HCOOCH=CHCH3��HCOOC��CH3��C=CH2��
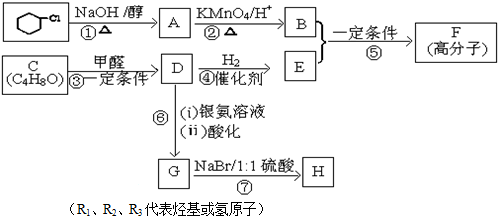
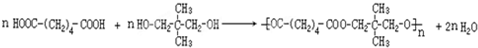
���� �������и�����ת����ϵ��A��ˮ�����ӳɷ�Ӧ��BΪCH3CHO��B������CΪCH3COOH������D�ķ���ʽ��֪��A��C�����ӳɷ�Ӧ��DΪCH3COOCH=CH2��D�����Ӿ۷�Ӧ��EΪ ��E��״�������Ϣ���е�ȡ����Ӧ����ʪ���������������M�Ľṹ��ʽ��֪�������ϩ�����ӳɷ�Ӧ��FΪ
��E��״�������Ϣ���е�ȡ����Ӧ����ʪ���������������M�Ľṹ��ʽ��֪�������ϩ�����ӳɷ�Ӧ��FΪ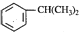 ��F�������������������·��������ϵ�ȡ����Ӧ��M���ݴ˴��⣮
��F�������������������·��������ϵ�ȡ����Ӧ��M���ݴ˴��⣮
��� �⣺�������и�����ת����ϵ��A��ˮ�����ӳɷ�Ӧ��BΪCH3CHO��B������CΪCH3COOH������D�ķ���ʽ��֪��A��C�����ӳɷ�Ӧ��DΪCH3COOCH=CH2��D�����Ӿ۷�Ӧ��EΪ ��E��״�������Ϣ���е�ȡ����Ӧ����ʪ���������������M�Ľṹ��ʽ��֪�������ϩ�����ӳɷ�Ӧ��FΪ
��E��״�������Ϣ���е�ȡ����Ӧ����ʪ���������������M�Ľṹ��ʽ��֪�������ϩ�����ӳɷ�Ӧ��FΪ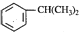 ��F�������������������·��������ϵ�ȡ����Ӧ��M��
��F�������������������·��������ϵ�ȡ����Ӧ��M��
��1����������ķ�����֪����Ӧ��Ϊȡ����Ӧ����Ӧ��Ϊ�ӳɷ�Ӧ����Ӧ�ݵķ�Ӧ����Ϊ������������
�ʴ�Ϊ��ȡ����Ӧ���ӳɷ�Ӧ��������������
��2��B����C��Ӧ����ʽΪ2CH3CHO+O2$��_{��}^{����}$2CH3COOH����Ӧ�ڵķ���ʽΪnCH3COOCH=CH2 $\stackrel{����}{��}$ ��
��
�ʴ�Ϊ��2CH3CHO+O2$��_{��}^{����}$2CH3COOH��nCH3COOCH=CH2 $\stackrel{����}{��}$ ��
��
��3���� �У�-C4H9�Ľṹ��-CH2CH2CH2CH3��-CH2CH��CH3��CH3��-CH��CH3��CH2CH3��-C��CH3��3����4�֣�����R�Ľṹ��4�֣�
�У�-C4H9�Ľṹ��-CH2CH2CH2CH3��-CH2CH��CH3��CH3��-CH��CH3��CH2CH3��-C��CH3��3����4�֣�����R�Ľṹ��4�֣�
�ʴ�Ϊ��4��
��4��DΪCH3COOCH=CH2����̼̼˫�����ܷ���������Ӧ����ȩ��������������D��ͬ���칹��Ľṹ��ʽΪHCOOCH2CH=CH2��HCOOCH=CHCH3��HCOOC��CH3��C=CH2��
�ʴ�Ϊ��HCOOCH2CH=CH2��HCOOCH=CHCH3��HCOOC��CH3��C=CH2��
���� ���⿼���л���ĺϳɣ���Ŀ�ѶȲ���ע������л���Ĺ����ŵ����ʣ�������ע��ͬ���칹�����д������ע����������Ϣ��

 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д� �߽�������ϵ�д�
�߽�������ϵ�д�| A�� | 0.10 | B�� | 0.20 | C�� | 0.30 | D�� | 0.40 |
| A�� | ������̼���ӵı���ģ��  | |
| B�� | �����������ͨʽ CnH2n-6��n��6�� | |
| C�� | 12C��14Cԭ�ӽṹʾ��ͼ���ɱ�ʾΪ 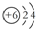 | |
| D�� | �ǻ��ĵ���ʽ 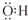 |
| A�� | �����ȴ����������� | |
| B�� | �����ĸ�ʴ������������Ĥ�����ܱ����ڲ���� | |
| C�� | ��������������ʴʱ��������ӦʽΪ��O2+2H2O+4e��4OH- | |
| D�� | Ϊ�������¸ֹܲ��ܸ�ʴ����ʹ����ֱ����Դ�������� |
| A�� | 0.01 mol/L��������Һ | B�� | 0.02 mol/L��CH3COOH��Һ | ||
| C�� | 0.02 mol/L��NaCl��Һ | D�� | 0.01 mol/L��K2SO4��Һ |
 ����A��D�����ǣ�������
����A��D�����ǣ���������C��O2 ��Na��O2 ��NaOH��CO2 ��S��O2 ��Fe��Cl2��
| A�� | �٢ڢ� | B�� | �ܢ� | C�� | �٢ڢ� | D�� | �٢ڢۢ� |
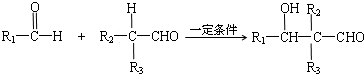
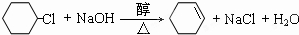 ��
��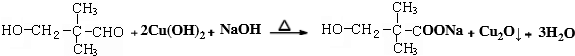
 ��
��