��Ŀ����
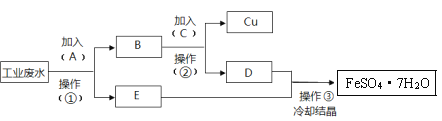
����Ŀ����1����ͼ�У�Ϊ�˼�����ˮ�Ը�բ��A�ĸ�ʴ������B����ѡ��_____������ĸ��ţ����õ绯ѧԭ�����Ͳ���B�趨�ڲ�ԭ��________��
a��̼�� b��п�� c��ͭ��
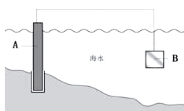
��2����ͼ����բ��C��__�������Ȼ�����Һģ�⺣ˮ����ʵ�飬DΪʯī�飬��D�ϵĵ缫��ӦʽΪ_______�����õ缫��Ӧ����ķ�����________��
��3��þȼ�ϵ���ڿ��ƶ������豸��Դ�ͱ��õ�Դ�ȷ���Ӧ��ǰ����������ͼΪ��þ-�������Ρ�ȼ�ϵ��ԭ��ʾ��ͼ���缫Ϊþ�Ͻ�Ͳ��Ͻ�
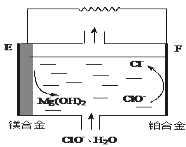
��EΪ��ȼ�ϵ�صļ�___���������������F�缫�ϵĵ缫��ӦʽΪ____��
��þȼ�ϵ�ظ����������Ը�ʴ����������ʹ���������ʽ��ͣ��û�ѧ�����ѧ����ʽ��������ԭ��__��
��4����ȩ�ᣨHOOC-CHO�����л��ϳɵ���Ҫ�м��塣��ҵ���á�˫���ҳɶԵ�ⷨ��������ȩ�ᣬԭ����ͼ��ʾ����װ��������������Ϊ���Ե缫�������Ҿ��ɲ�����ȩ�ᣬ�����Ҷ�ȩ��M�缫�IJ��ﷴӦ������ȩ�ᡣN���Ҷ���ֱ�ӷ�Ӧ������ȩ�ᡣ

��N�缫�ϵĵ缫��ӦʽΪ_________��
������2molH+ͨ�����ӽ���Ĥ������ȫ�����˷�Ӧ�����װ�������ɵ���ȩ��Ϊ______mol��
���𰸡�B п��ԭ��صĸ�������ʧ���ӣ�Zn-2e-=Zn2+�����������ܸ�ʴ���趨�ڲ� �� 2Cl��-2e��=Cl2�� ʪ��ĵ��۵⻯����ֽ����������������ֽ������֤��������������ȡ����������Һ�μӵ���KI��Һ�������� �� ClO��+2e��+H2O = Cl��+2OH�� Mg+2H2O= Mg(OH)2+H2�� HOOC-COOH+2e��+2H+=HOOC-CHO+ H2O 2
��������
(1)�γ�ԭ���ʱ��Fe����������������Ҫѡ������Ա�Feǿ�Ľ���������������ѡп��п�Ļ����Ա�Feǿ�����������������ܸ�ʴ���趨�ڲ�
�ʴ�Ϊ��b��п����ԭ��صĸ�����(ʧ���ӣ�Zn2e�TZn2+)���������ܸ�ʴ���趨�ڲ�
(2)Fe�����������������բ��C������������Ȼ�����Һʱ������������ʧ����������������缫��ӦΪ��2Cl2e�TCl2��������������ʪ��ĵ��۵⻯����ֽ������ʪ��ĵ��۵⻯����ֽ����������������ֽ������֤������������
�ʴ�Ϊ������2Cl2e�TCl2����ʪ��ĵ��۵⻯����ֽ����������������ֽ������֤������������
(3)����þ����������ȼ�ϵ����ʧ���ӵ�Ϊ����,��MgΪ����;������ClO�õ�������������,�������ĵ缫��ӦʽΪ��ClO+2e+H2O�TCl+2OH��
�ʴ�Ϊ������ClO+2e+H2O�TCl+2OH��
��Mg�Ļ����Խ�ǿ����ˮ��Ӧ�����������䷴ӦΪ��Mg+2H2O�TMg(OH)2+H2����
�ʴ�Ϊ��Mg+2H2O�TMg(OH)2+H2����
(4)��N�缫��HOOCCOOH�õ�������HOOCCHO����缫��ӦʽΪHOOCCOOH+2e+2H+�THOOCCHO+H2O��
�ʴ�Ϊ��HOOCCOOH+2e+2H+�THOOCCHO+H2O��
��2molH+ͨ�����ӽ���Ĥ��������ת��2mol���ӣ����ݵ缫����ʽHOOCCOOH+2e+2H+�THOOCCHO+H2O����֪����1mol��ȩ�ᣬ��������������ȩ�������������ɵ���ȩ��Ϊ2mol��
�ʴ�Ϊ��2��

����Ŀ��Na��Al��Fe��Cu����ѧ��ѧ����Ҫ�Ľ���Ԫ�ء����ǵĵ��ʼ��仯����֮���кܶ�ת����ϵ���±��������ʲ��ܰ���ͼ����������ʾһ����ɣ���ϵ�ת������ �� ��
ѡ�� | A | B | C | D |
|
a | Na | Al | Fe | Cu | |
b | NaOH | Al2O3 | FeCl3 | CuSO4 | |
c | NaCl | Al��OH��3 | FeCl2 | CuCl2 |
A. AB. BC. CD. D
����Ŀ��ijѧ����0.2000mol��L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������������£�
�� ������ˮϴ�Ӽ�ʽ�ζ��ܣ�������ע��NaOH��Һ����0���̶�����
�� �̶��õζ��ܲ�ʹ�ζ��ܼ������Һ��
������Һ������0������0���̶������£������¶���
����ȡ20.00mL����Һע��ྻ����ƿ�У�������2�η�̪��Һ
���ñ�Һ�ζ����յ㣬���µζ���Һ�������
��գ���1�����ϲ����д�����ǣ����ţ�_________���ô�������ᵼ�²ⶨ���__________��(����ƫ��������ƫС��������Ӱ����)
��2���������У��ڼ�¼�ζ���Һ�����ʱ���ζ��ܼ��������ݣ����²ⶨ���____________��(����ƫ��������ƫС��������Ӱ����)
��3���жϵζ��յ�������ǣ�___________________________________��
��4������ͼ��ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ_________mL

��5�������������ݣ���������������Һ��Ũ�ȣ�_______________________mol��L-1
�ζ����� | ����Һ���(mL) | ���ռ������mL�� | |
�ζ�ǰ���� | �ζ������ | ||
��һ�� | 20.00 | 0.40 | 20.40 |
�ڶ��� | 20.00 | 4.00 | 24.00 |
������ | 20.00 | 2.00 | 24.10 |