��Ŀ����
8��A��B�������ɼ����ȵIJ������ɣ�A���ݻ����䣻B���������������ƶ�����������ѹǿ��ȣ�����ͬ�����£���������NO2ѹ���ݻ���ͬ��A��B�������з������·�Ӧ��2NO2?N2O4��H��0���á�=��������������������գ���1����Ӧ��ʼʱ����N2O4������v��A��=v��B����
��2����Ӧ������N2O4������v��A����v��B����
��3��ƽ��ʱ�������з�������n��A����n��B����
��4���ﵽƽ��ʱ������ѹǿp��A����p��B����
���� ��1����Ӧ��ʼʱNO2��Ũ����ͬ�����Է�Ӧ������ȣ�
��2��2NO2?N2O4�������ʵ������ٵķ�Ӧ�����Է�Ӧ������B������������٣���ϵB��ѹǿ������ϵA��ѹǿ��
��3��2NO2?N2O4�������ʵ������ٵķ�Ӧ�����Է�Ӧ������B������������٣���ϵB��ѹǿ������ϵA��ѹǿ���ɴ˷�����
��4��2NO2?N2O4�������ʵ������ٵķ�Ӧ�����Է�Ӧ������B������������٣���ϵB��ѹǿ������ϵA��ѹǿ���ɴ˷������
��� �⣺��1����Ӧ��ʼʱNO2��Ũ����ͬ�����Է�Ӧ������ȣ�����v��A��=v��B�����ʴ�Ϊ��=��
��2��2NO2?N2O4�������ʵ������ٵķ�Ӧ�����Է�Ӧ������B������������٣���ϵB��ѹǿ������ϵA��ѹǿ����ΪѹǿԽ��Ӧ����Խ�죬����v��A����v��B�����ʴ�Ϊ������
��3��2NO2?N2O4�������ʵ������ٵķ�Ӧ�����Է�Ӧ������B������������٣���ϵB��ѹǿ������ϵA��ѹǿ������ѹǿ����������ٵķ����ƶ���������ϵB�з���С���ʴ�Ϊ������
��4��2NO2?N2O4�������ʵ������ٵķ�Ӧ�����Է�Ӧ������B������������٣���ϵB��ѹǿ������ϵA��ѹǿ������p��A����p��B�����ʴ�Ϊ������
���� ���⿼�黯ѧ��Ӧ���ʵ�Ӱ�����صȣ��ѶȲ���ע����Ŀʵ��Ϊ����������������������ѹǿ�ı仯�ǹؼ���

| A�� | CH3CH2NO2 | B�� | CH2�TCHBr | C�� | CH2Cl2 | D�� |  |
| A�� | ��ȥ��NH4��2CO3��Һ�к��е�����NH4HCO3���ʵķ����Ǽ���NaOH��Һ | |
| B�� | ������HCl��Cl2ͨ�뱥��NaHCO3��Һ�в��ܵõ����������� | |
| C�� | ������Fe2O3�Ĺ����зֱ����HCl��Һ��HI��Һ�����߷�Ӧ������ͬ | |
| D�� | ������м���������ŨHNO3���ȣ��ܵõ�ʹ����ʯ��ˮ����ǵ����壬֤���ù�����һ������CO32-��HCO3- |
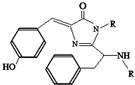 ���������ѧ��Ǯ�������ձ���ѧ�������������ѧ������•ɳ�������ڷ��ֺ��о���ɫӫ�⵰�ף�GFP����������ͻ������������2008��ŵ������ѧ�������о�����GFP�е���ɫ���Žṹ��ͼ��ʾ�������й�GFP��˵������ȷ�ģ�������
���������ѧ��Ǯ�������ձ���ѧ�������������ѧ������•ɳ�������ڷ��ֺ��о���ɫӫ�⵰�ף�GFP����������ͻ������������2008��ŵ������ѧ�������о�����GFP�е���ɫ���Žṹ��ͼ��ʾ�������й�GFP��˵������ȷ�ģ�������| A�� | ���л������ڷ����� | |
| B�� | ���л����ܷ���ˮ�ⷴӦ����1mol���л�����������������2mol | |
| C�� | ���л���������ˮ����ȡ����Ӧ����Br2 3mol | |
| D�� | ���л�����������ᷴӦ��������̼��������Һ��Ӧ |