��Ŀ����
����Ŀ����ҵ�����г���������NaCl��ijУ��ѧ����С�������ͼ��ʾװ�ã��ⶨ��ҵ������Na2CO3�ĺ�����
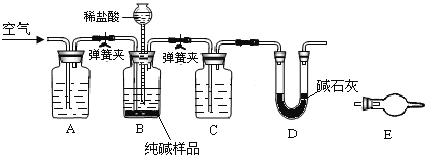
��1������װ��B�����Եķ����ǣ�����������©���������������н����ɼк�©��ע��һ������ˮ��ʹ©���ڵ�ˮ�����ƿ�ڵ�ˮ�棬ֹͣ��ˮ����________________��˵��װ�ò�©����
��2��װ��A��������____��װ��C�е��Լ�Ϊ___��
��3��ijͬѧ��Ϊ��Dװ�ú�Ӧ������Eװ�ã�װ���ʵ��Լ���������Ϊ�Ƿ��Ҫ��____��ѡ���Ҫ������Ҫ�������жϵ�������____________________��
��4��ʵ��ǰ��ȡ28.80g��Ʒ��ʵ�����Dװ������8.80g������Ʒ��Na2CO3����������Ϊ_____��
���𰸡�©�������Լ�ƿ�е�Һ���ֲ��ٱ仯��©���е�Һ�治���½���ȥ������CO2����ֹӰ��������Ũ�����Ҫ��Ϊװ��E�����տ����еĶ�����̼��ˮ������Ӱ��������73.6%
��������
��1����װ�ò�©��������Һ���Ӧ���ֲ��䣬�ʴ�Ϊ��©�������Լ�ƿ�е�Һ���ֲ��ٱ仯��©���е�Һ�治���½���
��2��װ��A�����տ����еĶ�����̼����ֹӰ��ʵ������װ��C��ʢװŨ�����ȥ������̼����ˮ�ݣ��ʴ�Ϊ����ȥ������CO2����ֹӰ����������Ũ������
��3����Dװ�ú�Ӧ������Eװ�ã��������ü�ʯ������ˮ�����Ͷ�����̼����������е�ˮ�����Ͷ�����̼��E���ղ������ʴ�Ϊ����Ҫ����Ϊװ��E�����տ����еĶ�����̼��ˮ������Ӱ����������
��4��Dװ������8.80g��������Ķ�����̼Ϊ8.8g��n��CO2��=8.8g��44g/mol=0.2mol����̼�غ�ɵ�n��Na2CO3��=0.2mol������Ʒ��Na2CO3����������Ϊ0.2mol��106g/mol��28.8g=73.6%���ʴ�Ϊ��73.6%��

����Ŀ������ʵ�����������ͽ��۾���ȷ���ǣ� ��
ѡ�� | ���� | ���� | ���� |
A | ��ij��Һ�м���NaOH ��Һ������ʪ��ĺ�ɫʯ����ֽ���� | ��ֽû�б��� | ԭ��Һ��û����NH4+ |
B | ���մ��С�մ���Һ�зֱ�������� | ��ð���� | ���߾��������ᷴӦ |
C | ��ij��Һ�еμ���ˮ���ټ�KSCN��Һ | ��Һ��� | ԭ��Һ��һ����Fe2+ |
D | ��ij��ɫ��Һ�еμ�BaCl2��Һ | ������ɫ���� | ����ɫ��Һ��һ������SO42- |
A. A B. B C. C D. D