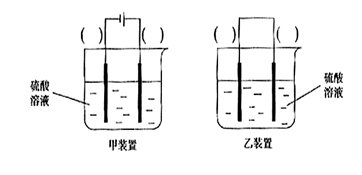
��Ŀ����
��11�֣������������ʹ�õĽ���֮һ�����������й�֪ʶ���ش��������⣺
��1�����йر�����Ŀǰ����ұ�����ȸߴ�99.9999%���������ڴ��������������У���ȷ���ǣ� ��
A��Ӳ�ȱ������� B����ʴ��ǿ����������
C�����������ᷴӦ D���벻��ֳɷ���ͬ E�������Ũ�����жۻ�
��2�����ˮ����εμ�1mol��L-1FeC13��Һ����Һ������ĺ��ɫ���γɸ÷�ɢϵ�������ȷ�Χ�� ��
��3�����ӹ�ҵ����30%��FeC13��Һ��ʴ���ھ�Ե���ϵ�ͭ������ӡˢ��·�塣��д��
FeC13��Һ��ͭ��Ӧ�����ӷ���ʽ �����鷴Ӧ�����Һ�л�����Fe3+���Լ���
��3�֣�����ÿ��2�֣���
��4�����Ӹ�ʴ��ķ�Һ�л���ͭ�����»��FeC13��Һ�����������Լ���������ˮ �����ۢ�Ũ�����Ũ������ռ��Ũ��ˮ����������Ҫ�õ���һ���Լ��� �� ��
A���٢ڢܢ� B���٢ۢܢ� C���ڢܢ� D���٢ܢޢ�
��1�����йر�����Ŀǰ����ұ�����ȸߴ�99.9999%���������ڴ��������������У���ȷ���ǣ� ��
A��Ӳ�ȱ������� B����ʴ��ǿ����������
C�����������ᷴӦ D���벻��ֳɷ���ͬ E�������Ũ�����жۻ�
��2�����ˮ����εμ�1mol��L-1FeC13��Һ����Һ������ĺ��ɫ���γɸ÷�ɢϵ�������ȷ�Χ�� ��
��3�����ӹ�ҵ����30%��FeC13��Һ��ʴ���ھ�Ե���ϵ�ͭ������ӡˢ��·�塣��д��
FeC13��Һ��ͭ��Ӧ�����ӷ���ʽ �����鷴Ӧ�����Һ�л�����Fe3+���Լ���
��3�֣�����ÿ��2�֣���
��4�����Ӹ�ʴ��ķ�Һ�л���ͭ�����»��FeC13��Һ�����������Լ���������ˮ �����ۢ�Ũ�����Ũ������ռ��Ũ��ˮ����������Ҫ�õ���һ���Լ��� �� ��
A���٢ڢܢ� B���٢ۢܢ� C���ڢܢ� D���٢ܢޢ�
��11�֣���1��BE (2)10-9��10-7 nm ��3��2Fe3++Cu=2Fe2++ Cu2+��ȡ��Ӧ�����Һ������������KSCN��Һ�������Һ���Ѫ��ɫ��˵����Һ����Fe3+���ڡ���4��A
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
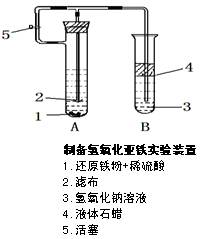
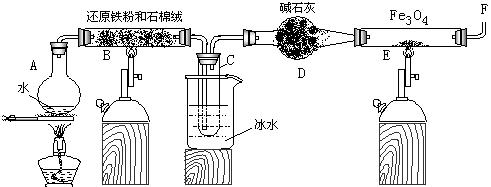
 Fe3O4 +4H2��һ��������ʵ����Ϊ���淴Ӧ,��ʵ���������ʵ��Fe��Fe3O4��ת���?���û�ѧƽ���ƶ�ԭ�����Խ���
Fe3O4 +4H2��һ��������ʵ����Ϊ���淴Ӧ,��ʵ���������ʵ��Fe��Fe3O4��ת���?���û�ѧƽ���ƶ�ԭ�����Խ���  2Cu+SO2
2Cu+SO2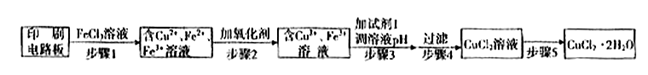 ��֤������I����FeCl3��Һ�����ķ����� ��
��֤������I����FeCl3��Һ�����ķ����� �� O2��g��==H2O��l�� ��H3=-286kJ/mol
O2��g��==H2O��l�� ��H3=-286kJ/mol