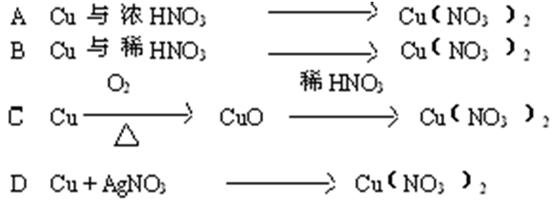
��Ŀ����
(18��)��һ��������,����ͨ���ۺ�ˮ������Ӧ,���Եõ�����������,���������ֿ��Ծ��˷�Ӧ���淴Ӧ,���ɿ�����ϸ�����ۡ�ijУ��ѧС��������ͼ����װ�ý�������ˮ��Ӧ��ʵ��,�����ò����һ����������������Ӧ��ȡ���ۡ�(װ���е�����̨�����еȱ�Ҫ�ļг�������ͼ�о�����ȥ)
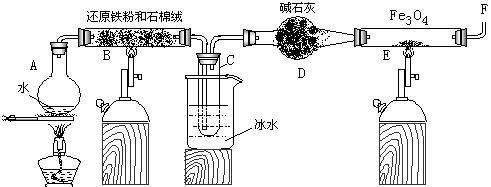
(1) ��ƿA�г��������Ƭ,�������� ,
С�Թܣõ������� ��
(2) Ϊ�˰�ȫ,�ڣŹ��еķ�Ӧ����ǰ,��F���ڴ����� ,
�Ź��еķ�Ӧ��ʼ��, ��F���ڴ�Ӧ ��
(3) ��Ӧ3Fe +4H2O(g) Fe3O4 +4H2��һ��������ʵ����Ϊ���淴Ӧ,��ʵ���������ʵ��Fe��Fe3O4��ת���?���û�ѧƽ���ƶ�ԭ�����Խ���
Fe3O4 +4H2��һ��������ʵ����Ϊ���淴Ӧ,��ʵ���������ʵ��Fe��Fe3O4��ת���?���û�ѧƽ���ƶ�ԭ�����Խ���
��
(4) ֹͣ��Ӧ,��B����ȴ��,ȡ���й���������ϡ����,��ַ�Ӧ����ˡ�����֤����Һ��
����Fe3+��ʵ�����������
��

(1) ��ƿA�г��������Ƭ,�������� ,
С�Թܣõ������� ��
(2) Ϊ�˰�ȫ,�ڣŹ��еķ�Ӧ����ǰ,��F���ڴ����� ,
�Ź��еķ�Ӧ��ʼ��, ��F���ڴ�Ӧ ��
(3) ��Ӧ3Fe +4H2O(g)
 Fe3O4 +4H2��һ��������ʵ����Ϊ���淴Ӧ,��ʵ���������ʵ��Fe��Fe3O4��ת���?���û�ѧƽ���ƶ�ԭ�����Խ���
Fe3O4 +4H2��һ��������ʵ����Ϊ���淴Ӧ,��ʵ���������ʵ��Fe��Fe3O4��ת���?���û�ѧƽ���ƶ�ԭ�����Խ��� ��
(4) ֹͣ��Ӧ,��B����ȴ��,ȡ���й���������ϡ����,��ַ�Ӧ����ˡ�����֤����Һ��
����Fe3+��ʵ�����������
��
(18��)
(1) ��ֹҺ�屩�� (2��) ����ˮ����(2��)
(2) ���������Ĵ���(2��) ��ȼ����(2��)
(3) ʵ���в���ͨ��ˮ��������Ӧ��Ũ��,������������С������Ũ��,�Ӷ�ʹ��Ӧ�������(2��)
(4) ȡ��Һ����,�����еμ�KSCN��Һ, ��Һ��ΪѪ��ɫ,��֤������Fe3+(2��)
(1) ��ֹҺ�屩�� (2��) ����ˮ����(2��)
(2) ���������Ĵ���(2��) ��ȼ����(2��)
(3) ʵ���в���ͨ��ˮ��������Ӧ��Ũ��,������������С������Ũ��,�Ӷ�ʹ��Ӧ�������(2��)
(4) ȡ��Һ����,�����еμ�KSCN��Һ, ��Һ��ΪѪ��ɫ,��֤������Fe3+(2��)
��

��ϰ��ϵ�д�
�����Ŀ
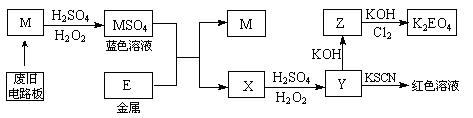
 ��2��M MSO4�����У��������� ����ԭ���� ��
��2��M MSO4�����У��������� ����ԭ���� �� �ٿ�?
�ٿ�?