��Ŀ����
��12�֣�����������FeSO4��7H2O)��һ����Ҫ��ʳƷ���������Ӽ���ʵ����ͨ������ʵ���ɷ���м�Ʊ�FeSO4��7H2O���壺
�ٽ�5% Na2CO3��Һ���뵽ʢ��һ��������м���ձ��У����������ӣ�����������ȥNa2CO3��Һ��Ȼ����м��ˮϴ��2��3�飻����ϴ�ӹ��ķ���м�м��������ϡ���ᣬ�����¶���50��80 ��֮������м�ľ����۳��ȹ��ˣ�����Һת�뵽�ܱ������У����á���ȴ�ᾧ���ܴ��ᾧ��Ϻ��˳����壬��������ˮϴ��2��3�Σ�������ֽ���������ɣ��ݽ��Ƶõ�FeSO4��7H2O�������һ��С���ƿ�У��ܱձ��档
������������⣺
��1��ʵ�鲽��ٵ�Ŀ����___________________�����ȵ�������
_________________________��
��2��ʵ�鲽������Բ�������������
________________________________________________��
��3��ʵ�鲽�������������ˮϴ�Ӿ��壬��Ŀ����_________________��
__________________��
��4�����������Ϻ��֣����������ڲ�ͬ�¶��½ᾧ�ɷֱ�õ�FeSO4��7H2O��FeSO4��4H2O��FeSO4��H2O�����������ڲ�ͬ�¶��µ��ܽ�Ⱥ��¶������������������±���ʾ������56.7 �桢64 ���¶��¿�ͬʱ�������־��壩��

�����������ܽ�Ⱥ�������������
����ݱ����������������������ܽ�����ߡ�
��5�����������������Һ�нᾧ��FeSO4��4H2O��Ӧ���ƵĽᾧ�¶ȣ�t���ķ�ΧΪ________��
�ٽ�5% Na2CO3��Һ���뵽ʢ��һ��������м���ձ��У����������ӣ�����������ȥNa2CO3��Һ��Ȼ����м��ˮϴ��2��3�飻����ϴ�ӹ��ķ���м�м��������ϡ���ᣬ�����¶���50��80 ��֮������м�ľ����۳��ȹ��ˣ�����Һת�뵽�ܱ������У����á���ȴ�ᾧ���ܴ��ᾧ��Ϻ��˳����壬��������ˮϴ��2��3�Σ�������ֽ���������ɣ��ݽ��Ƶõ�FeSO4��7H2O�������һ��С���ƿ�У��ܱձ��档
������������⣺
��1��ʵ�鲽��ٵ�Ŀ����___________________�����ȵ�������
_________________________��
��2��ʵ�鲽������Բ�������������
________________________________________________��
��3��ʵ�鲽�������������ˮϴ�Ӿ��壬��Ŀ����_________________��
__________________��
��4�����������Ϻ��֣����������ڲ�ͬ�¶��½ᾧ�ɷֱ�õ�FeSO4��7H2O��FeSO4��4H2O��FeSO4��H2O�����������ڲ�ͬ�¶��µ��ܽ�Ⱥ��¶������������������±���ʾ������56.7 �桢64 ���¶��¿�ͬʱ�������־��壩��

�����������ܽ�Ⱥ�������������
| �¶�/�� | 0 | 10 | 30 | 50 | 56.7 | 60 | 64 | 70 | 80 | 90 |
| �ܽ��/g | 14.0 | 17.0 | 25.0 | 33.0 | 35.2 | 35.3 | 35.6 | 33.0 | 30.5 | 27.0 |
| �������� | FeSO4��7H2O | FeSO4��4H2O | FeSO4��H2O | |||||||
��5�����������������Һ�нᾧ��FeSO4��4H2O��Ӧ���ƵĽᾧ�¶ȣ�t���ķ�ΧΪ________��
��1�������� �����¶ȣ���Һ������ǿ��ȥ����������ǿ
��2��Ӧ����м��������Ӧ����Һ�б�������ʣ�ࣩ��������Һ�п�����Fe3+����
��3��ϴ�ӳ�ȥ������渽�ŵ����������
�ñ�ˮϴ�ӿɽ���ϴ�ӹ�����FeSO4��7H2O�����
��4����ͼ
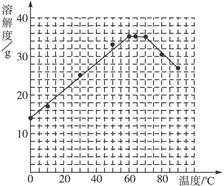
��5��56.7 ��<t<64 ��
��2��Ӧ����м��������Ӧ����Һ�б�������ʣ�ࣩ��������Һ�п�����Fe3+����
��3��ϴ�ӳ�ȥ������渽�ŵ����������
�ñ�ˮϴ�ӿɽ���ϴ�ӹ�����FeSO4��7H2O�����
��4����ͼ

��5��56.7 ��<t<64 ��
(1)Na2CO3��Һ����Ҫ�����dz�ȥ����м��������ۣ���Ϊ�����¶ȣ�Na2CO3��ˮ��̶ȴ�ȥ������ǿ��������ȡ�
��2��Fe2+�ױ������е�O2������Fe3+��Ҫ��֤�õ��ϴ�����FeSO4��7H2O���壬�������ܽ���мʱ��֤��м������
��3��Ҫ�õ�������FeSO4��7H2O���壬���˳���������ϴ��2��3�Σ��Գ�ȥ�������������ӡ�
��2��Fe2+�ױ������е�O2������Fe3+��Ҫ��֤�õ��ϴ�����FeSO4��7H2O���壬�������ܽ���мʱ��֤��м������
��3��Ҫ�õ�������FeSO4��7H2O���壬���˳���������ϴ��2��3�Σ��Գ�ȥ�������������ӡ�

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д� ����˼ά����ѵ����ʱ��ѧ��ϵ�д�
����˼ά����ѵ����ʱ��ѧ��ϵ�д�
�����Ŀ
 Fe(OH)2+2H+����������������Һ�м������ᣬ�۲쵽�������ǣ� ��
Fe(OH)2+2H+����������������Һ�м������ᣬ�۲쵽�������ǣ� ��