��Ŀ����
12���������ӷ���ʽ��ȷ���ǣ�������| A�� | �����ʹ���Ը��������Һ��ɫ�������ӷ���ʽΪ��2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O | |
| B�� | ��NH4Al��SO4��2��Һ�м��������Ba��OH��2ϡ��Һ��NH4++Al3++2SO42-+2Ba2++5OH-=2BaSO4��+NH3•H2O+AlO2-+2H2O | |
| C�� | NaHSO3��Һ��FeCl3��Ӧ�����ӷ���ʽ��SO32-+2Fe3++H2O=SO42-+2Fe2++2H+ | |
| D�� | NH4HCO3��Һ�м�������NaOH��NH4++OH-�TNH3•H2O |
���� A������������ԭ��Ӧ����ѭ���ӡ�����غ㣬���������ӷ�Ӧ�б�����ѧʽ��
B�����������Ba��OH��2ϡ��Һ����Ӧ�������ᱵ��һˮ�ϰ���ƫ���ᱵ��ˮ��
C������������ԭ��Ӧ�������ӷ�Ӧ��HSO3-���ܲ�֣�
D����������NaOH��笠����Ӳ���Ӧ��
��� �⣺A�������ʹ���Ը��������Һ��ɫ������������ԭ��Ӧ�����ӷ���ʽΪ2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O����A����
B����NH4Al��SO4��2��Һ�м��������Ba��OH��2ϡ��Һ��NH4++Al3++2SO42-+2Ba2++5OH-=2BaSO4��+NH3•H2O+AlO2-+2H2O����B��ȷ��
C��NaHSO3��Һ��FeCl3��Ӧ�����ӷ���ʽΪHSO3-+2Fe3++H2O=SO42-+2Fe2++3H+����C����
D��NH4HCO3��Һ�м�������NaOH�����ӷ�ӦΪHCO3-+OH-�TCO32-+H2O����D����
��ѡB��
���� ���⿼�����ӷ�Ӧ����ʽ��д�������жϣ�Ϊ��Ƶ���㣬���շ����ķ�Ӧ�����ӷ�Ӧ����д����Ϊ���Ĺؼ������������йص����ӷ�Ӧ��������ԭ��Ӧ�����ӷ�Ӧ���飬ע�����ӷ�Ӧ�б�����ѧʽ�����ʣ���Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
2����a mol C2H4��b mol H2������ܱ������У����ʵ������£���Ӧ�ﵽƽ��ʱ����p molC2H6����������ƽ����������ȫȼ�����ɶ�����̼��ˮ����Ҫ���������ǣ�������
| A�� | ��3a+b��mol | B�� | ��3a+$\frac{b}{2}$��mol | C�� | ��3a+3p+$\frac{b}{2}$��mol | D�� | ��3a+$\frac{b}{2}$-3p��mol |
17�� �Ҵ������в�ͬ�Ļ�ѧ����ͼ��ʾ�����Ҵ��ڸ��ַ�Ӧ��Ӧ���ѵļ�˵������ȷ���ǣ�������
�Ҵ������в�ͬ�Ļ�ѧ����ͼ��ʾ�����Ҵ��ڸ��ַ�Ӧ��Ӧ���ѵļ�˵������ȷ���ǣ�������
 �Ҵ������в�ͬ�Ļ�ѧ����ͼ��ʾ�����Ҵ��ڸ��ַ�Ӧ��Ӧ���ѵļ�˵������ȷ���ǣ�������
�Ҵ������в�ͬ�Ļ�ѧ����ͼ��ʾ�����Ҵ��ڸ��ַ�Ӧ��Ӧ���ѵļ�˵������ȷ���ǣ�������| A�� | �����������ʱ�����ٶ� | |
| B�� | Ũ���Ṳ����170��ʱ�����ں͢ݶ��� | |
| C�� | �����ᡢŨ���Ṳ��ʱ�����ڶ��� | |
| D�� | �������º�������Ӧʱ�����ٺܶ͢��� |
4��ͼΪʵ���ҴӺ�������ȡ�ⵥ�ʵ�����ʾ��ͼ���ж�����˵��������ǣ�������
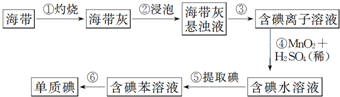

| A�� | �������Ҫ�õ������� | B�� | �������Ҫ����װ�� | ||
| C�� | �������Ҫ�õ���Һ©�� | D�� | �������Ҫ����װ�� |
7��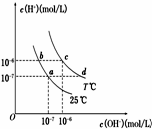 �ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-������ͼ��ʾ��ϵ�����������������ӹ���˵������ȷ���ǣ�������
�ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-������ͼ��ʾ��ϵ�����������������ӹ���˵������ȷ���ǣ�������
 �ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-������ͼ��ʾ��ϵ�����������������ӹ���˵������ȷ���ǣ�������
�ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-������ͼ��ʾ��ϵ�����������������ӹ���˵������ȷ���ǣ�������| A�� | a���Ӧ����Һ�д������ڣ�Fe3+��Ba2+��K+��NO3- | |
| B�� | b���Ӧ����Һ�д������ڣ�Na+��K+��NH4+��Cl- | |
| C�� | d���Ӧ����Һ�д������ڣ�Na+��Ba2+��Cl-��Al3+ | |
| D�� | c���Ӧ����Һ�д������ڣ�CO32-��Na+��Cu2+��SO42- |

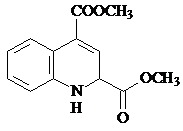 ��
�� ��
��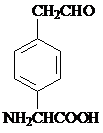 ��
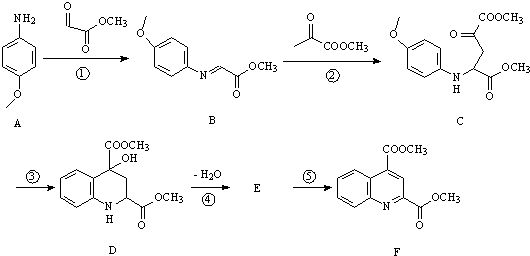
��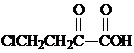 �ͱ����Ҵ�Ϊԭ�ϣ��ɺϳ�
�ͱ����Ҵ�Ϊԭ�ϣ��ɺϳ�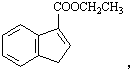 ��д���ϳ�����
��д���ϳ�����
 ��ҵ���о�����CO2�������״�ȼ�ϵķ������÷������Ȼ�ѧ����ʽ�ǣ�
��ҵ���о�����CO2�������״�ȼ�ϵķ������÷������Ȼ�ѧ����ʽ�ǣ�