��Ŀ����
10���±���Ԫ�����ڱ���һ���֣�������ŷֱ����ijһԪ�أ���ش��������⣮| ����/�� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | �� | �� | �� | �� | ||||
| �� | �� | �� | �� | �� | �� | �� |
��2���ۡ��ۡ��ݣ���ԭ�Ӱ뾶�ɴ�С��˳����Na��N��F��дԪ�ط��ţ���
��3���ࡢ����⻯���ԭ�Ը�ǿ����H2S���ѧʽ����
��4���١��������������ˮ�����У�������ǿ����HClO4���ѧʽ����������ǿ����NaOH���ѧʽ��������ˮ��Һ��ߵ�����������ˮ���ﷴӦ���������ӷ���ʽΪOH-+Al��OH��3=AlO2-+2H2O��
���� ��Ԫ�������ڱ���λ�ã���֪��ΪB����ΪC����ΪN����ΪF����ΪNa����ΪAl����ΪSi����ΪS����ΪCl����ΪArԪ�أ�
��1��ͬ����������ҽ����Լ������ǽ�������ǿ��ͬ�������϶��½�������ǿ���ǽ����Լ�����̼Ԫ������л���γɻ�����������ࣻNԪ�ص���̬�⻯����������������ˮ�����ֱ�ӻ���������Σ�
��2��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����
��3���ǽ�����Խǿ���⻯�ﻹԭ��Խ����
��4��������Ϊ��������������ǿ�����������Na�Ľ�������ǿ��NaOH�ļ�����ǿ��������������Ӧ����ƫ�����ƺ�ˮ��
��� �⣺��Ԫ�������ڱ���λ�ã���֪��ΪC����ΪN����ΪO����ΪF����ΪNa����ΪAl����ΪSi����ΪS����ΪCl����ΪAr��
��1��ͬ����������ҽ����Լ������ǽ�������ǿ��ͬ�������϶��½�������ǿ���ǽ����Լ�����������Ԫ����Na�Ľ�������ǿ��FԪ�طǽ�������ǿ��̼Ԫ������л���γɻ�����������ࣻNԪ�ص���̬�⻯����������������ˮ�����ֱ�ӻ���������Σ�
�ʴ�Ϊ��Na��F��C��N��
��2����ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����ԭ�Ӱ뾶��Na��N��F��
�ʴ�Ϊ��Na��N��F��
��3���ǽ�����Cl��S���ǽ�����Խǿ����̬�⻯�ﻹԭ��Խ������ԭ�ԣ�H2S��HCl���ʴ�Ϊ��H2S��
��4��������Ϊ��������������ǿ���ᣬ����Ļ�ѧʽΪHClO4����������Na�Ľ�������ǿ�����Ӧ��NaOH�ļ�����ǿ��NaOH������������Ӧ����ƫ�����ƺ�ˮ�����ӷ�ӦΪOH-+Al��OH��3=AlO2-+2H2O��
�ʴ�Ϊ��HClO4��NaOH��OH-+Al��OH��3=AlO2-+2H2O��
���� ���⿼��Ԫ�����ڱ���Ԫ��������Ӧ�ã���Ҫѧ����������Ԫ�����ڱ��Ľṹ��Ԫ�ط��ţ�ע���Ԫ�������ɵ��������գ������ڻ���֪ʶ�Ĺ��̣�

| A�� | CO2ͨ�뵽CaCl2��Һ�� | |
| B�� | NH3ͨ��AlCl3��Һ�� | |
| C�� | ����֬�ް���Na2O2��ĩ¶���ڳ�ʪ������ | |
| D�� | ϡ����μӵ�Fe��OH��3������Һ�� |
| A�� | �Ȼ��ƵĻ�ѧʽ��CaCl | |
| B�� | þ���ӽṹʾ��ͼ��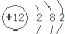 | |
| C�� | ̼���Ƶĵ��뷽��ʽ��Na2CO3�TNa++CO32- | |
| D�� | ���������ĵ��뷽��ʽBa��OH��2�TBa2++2OH- |
| A�� | һ������CO2 | B�� | һ������HBr�����ܺ���CO2 | ||
| C�� | һ������Cl2��CO2 | D�� | ���ܺ���CO2��Cl2 |
| A�� | 2d1 | B�� | 3f7 | C�� | 6s3 | D�� | 7p2 |
| A�� | �ӳɡ���ȥ��ȡ�� | B�� | ��ȥ���ӳɡ�ȡ�� | C�� | ȡ������ȥ���ӳ� | D�� | ȡ�����ӳɡ���ȥ |
| A�� | ��ȥ��Ӧ���ӳɷ�Ӧ | B�� | �ӳɷ�Ӧ����ȥ��Ӧ | ||
| C�� | ȡ����Ӧ���ӳɷ�Ӧ | D�� | �ӳɷ�Ӧ��ȡ����Ӧ |
| A�� | ͨ�������ᾧϡ����ķ�ʽ���Գ�ȥ�����NaCl��Na2CO3���е�Na2CO3�����ᴿ�õ�NaCl | |
| B�� | ͨ����ȼO2�ķ�ʽ���Գ�ȥ�����CO2��CO���е�CO�����ᴿ�õ�CO2 | |
| C�� | ͨ������ϡ����ķ�ʽ���Գ�ȥ�����Zn��Ag���е�Ag�����ᴿ�õ�Zn | |
| D�� | ͨ������ķ�ʽ���Գ�ȥ���������ˮ��Cl-���е�Cl-���Ӷ��ᴿ�õ�����ˮ |