��Ŀ����
����Ŀ����֪ʵ������ȡCl2�Ļ�ѧ����ʽΪ��4HCl��Ũ�� + MnO2 ![]() MnCl2 + Cl2�� + 2H2O���Իش��������⣺
MnCl2 + Cl2�� + 2H2O���Իش��������⣺
��1����ͼΪ��ȡCl2�ķ���װ�á�
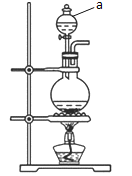
������a��������____��
������a��ʢ�ŵ��Լ�Ϊ____��
��2����ͼΪCl2���ռ���β������װ�á�

�ٸ������ռ�����Ϊ_____��
A ���ſ����� B ���ſ�����
���ձ��з�����Ӧ�Ļ�ѧ����ʽΪ_________��
��3�����Ƶñ�״����2.24L Cl2��������ҪMnO2������Ϊ___g��
���𰸡���Һ©�� Ũ�����HCl��Ũ�� A Cl2 + 2NaOH = NaCl + NaClO + H2O 8.7
��������
��1���ٸ���װ��ͼ�ж��������ƣ�
������aװҺ���Լ���
��2�����������ܶȱȿ�����
���ձ������������������������Ȼ��ơ��������ƺ�ˮ��
��3�����ݷ�Ӧ����ʽ������ҪMnO2��������
��1���ٸ���װ��ͼ��֪������a�Ƿ�Һ©����
������aװҺ���Լ�����������a��ʢ�ŵ��Լ�ΪŨ���
��2�����������ܶȱȿ������������ſ������ռ�����ѡA��
���ձ������������������������Ȼ��ơ��������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽ��Cl2 + 2NaOH = NaCl + NaClO + H2O��
��3����״����2.24L Cl2�����ʵ�����![]() ������ҪMnO2������Ϊx
������ҪMnO2������Ϊx

![]() 8.7g��
8.7g��

 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�