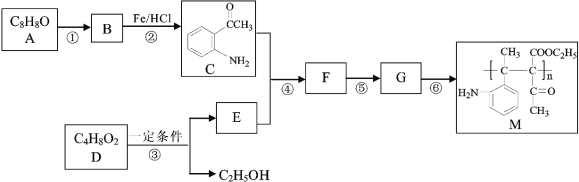
��Ŀ����
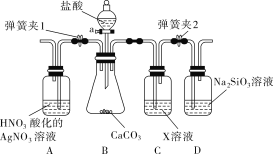
����Ŀ��ijͬѧΪ����֤̼������Ԫ�طǽ����Ե����ǿ��������ͼ��ʾװ�ý���ʵ��(�г���������ȥ���������Ѽ���)��
ʵ��������裺
�����ɼ�1���رյ��ɼ�2��������a���μ����ᡣ
��A�п�����ɫ����ʱ�������رջ���a��
��ش�
(1)B�з�Ӧ�����ӷ���ʽ��___________��
(2)ͨ��������������֪������е�������________(����ĸ)��
A.�ӷ��� B.��ԭ�� C.������ D.����
(3)Cװ�õ�������__________��X��________(д��ѧʽ)��
(4)Ϊ����֤̼�ķǽ�����ǿ�ڹ裬��������δд�IJ�����������___________��
(5)D�з�Ӧ�Ļ�ѧ����ʽ��________________��
���𰸡�CaCO3��2H��=Ca2����CO2����H2O A��D ��HCl���� NaHCO3 �رյ��ɼ�1�����ɼ�2����D�г��ְ�ɫ��״������ Na2SiO3��CO2��H2O=H2SiO3����Na2CO3
��������
��֤̼������Ԫ�طǽ����Ե����ǿ������Ҫ������ǿ���������ԭ���������̼��Ʒ�Ӧ�����Ȼ��ơ�������̼��ˮ�����������ӷ�����˶�����̼ͨ�������֮ǰ��Ҫ���ӷ�����HCl������һ����̼��������Һ������
(1)B����̼��ƺ�ϡ���ᷴӦ�����Ȼ��ơ�������̼��ˮ���䷴Ӧ�����ӷ���ʽ��CaCO3��2H��=Ca2����CO2����H2O���ʴ�Ϊ��CaCO3��2H��=Ca2����CO2����H2O��
(2)ͨ��������������A�п�����ɫ����˵��������лӷ��ԣ�������̼��Ʒ�Ӧ�����Ȼ��ơ�������̼��ˮ��˵������������ԣ���˵�֪������е�������AD���ʴ�Ϊ��AD��
(3)����������лӷ��ԣ��������Cװ�ó����ӷ�����HCl��X�Լ�Ϊ����̼������(NaHCO3)��Һ���ʴ�Ϊ����HCl���壻NaHCO3��
(4)Ϊ����֤̼�ķǽ�����ǿ�ڹ裬���ɵĶ�����̼ͨ�뵽��������Һ�У���˲�������δд�IJ����������ǹرյ��ɼ�1�����ɼ�2����D�г��ְ�ɫ��״�����ʴ�Ϊ���رյ��ɼ�1�����ɼ�2����D�г��ְ�ɫ��״������
(5)D�ж�����̼��������Һ��Ӧ���ɹ����̼���ƣ��䷴Ӧ�Ļ�ѧ����ʽ��Na2SiO3��CO2 ��H2O=H2SiO3����Na2CO3���ʴ�Ϊ��Na2SiO3��CO2��H2O=H2SiO3����Na2CO3��

����Ŀ���й���ѧ���ý����ƺ�![]() ��һ���������Ƶ��˽��ʯ��
��һ���������Ƶ��˽��ʯ��![]() �����ʯ��
�����ʯ��
�� |
| ���ʯ | ʯī | |
�۵㣨�棩 | 97.8 | 851 | 3550 | 3850 |
�е㣨�棩 | 882.9 | 1850���ֽ���� | ���� | 4250 |
(1)����Ӧ�ڳ�ѹ��890���½��У�д���÷�Ӧ��ƽ�ⳣ������ʽ____����![]() ����________��ѡ����ţ���
����________��ѡ����ţ���
a.��Ӧ�϶��ﵽƽ�� b.��Ӧ���ܴﵽƽ�� c.��Ӧ�϶�δ��ƽ��
(2)����Ӧ��10L�ܱ���������ѹ�½��У�5min�ڣ���ý��ʯ������������6g����ʱ�����![]() ________������Ӧ�¶���890�����ߵ�1860�棬�������������ƽ����Է���������________��ѡ����������������С����������������
________������Ӧ�¶���890�����ߵ�1860�棬�������������ƽ����Է���������________��ѡ����������������С����������������
(3)��Ӧ�л���ʯī���ɣ���֪��![]() ���������¶ȣ����ɵ�̼�����У����ʯ�ĺ�����������÷�Ӧ������Ӧ��________��Ӧ��������������������������
���������¶ȣ����ɵ�̼�����У����ʯ�ĺ�����������÷�Ӧ������Ӧ��________��Ӧ��������������������������