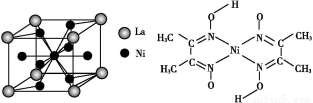
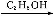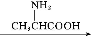ћвƒњƒЏ»Ё
Ґс.ЌЉ±нЈ®°ҐЌЉѕсЈ® «≥£”√µƒњ∆—І—–ЊњЈљЈ®°£
(1)ґћ÷№∆Џƒ≥÷ч„е‘™ЋЎMµƒµзјлƒ№«йњц»зЉ„ЌЉЋщ Њ,‘тM‘™ЋЎќї”Џ÷№∆Џ±нµƒµЏ°°°°°°°°„е°£
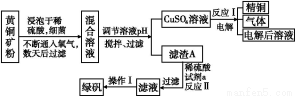
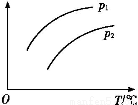
(2)““ЌЉ «—–Њњ≤њЈ÷‘™ЋЎµƒ«вїѓќпµƒЈ–µг±дїѓєж¬…µƒЌЉѕс,’џѕяcњ…“‘±ніп≥цµЏ°°°°°°°°„е‘™ЋЎ«вїѓќпµƒЈ–µгµƒ±дїѓєж¬…°£ЅљќїЌђ—Іґ‘ƒ≥÷ч„е‘™ЋЎ«вїѓќпµƒЈ–µгµƒ±дїѓ«ч ∆ї≠≥цЅЋЅљћх’џѕяaЇЌb,ƒг»ѕќ™’э»Јµƒ «°°°°°°°°°£
Ґт.”…—хїѓќпЊ≠¬»їѓ„ч”√…ъ≥…¬»їѓќп «є§“µ…ъ≤ъ¬»їѓќпµƒ≥£”√ЈљЈ®,Cl2°ҐCCl4 «≥£”√µƒ¬»їѓЉЅ°£»з:2Na2O+2Cl2 4NaCl+O2;2CaO+2Cl2
4NaCl+O2;2CaO+2Cl2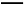 2CaCl2+O2;SiO2+2CCl4
2CaCl2+O2;SiO2+2CCl4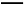 SiCl4+2COCl2;Cr2O3+3CCl4
SiCl4+2COCl2;Cr2O3+3CCl4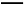 2CrCl3+3COCl2°£
2CrCl3+3COCl2°£
«лїЎірѕ¬Ѕ–ќ ћв:
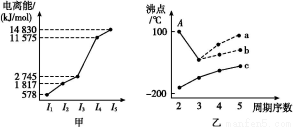
(1)Cr2O3°ҐCrCl3÷–CrЊщќ™+3Љџ,–і≥цCr3+µƒїщћђµз„”≈≈≤Љ љ:°° °£
(2)COCl2Ћ„≥∆єв∆ш,Ј÷„”÷–C‘≠„”≤…»°sp2‘”їѓ≥…Љь°£єв∆шЈ÷„”љбєє љ «°°°°°°°°°°°°°°°°,∆д÷–ћЉ—х‘≠„”÷ЃЉдє≤ЉџЉь «°°°°°°°°°£
A.2Єц¶“ЉьB.2Єц¶–Љь
C.1Єц¶“љ°,1Єц¶–Љь
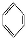
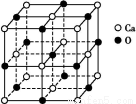
(3)CaOЊІ∞ы»зЌЉЋщ Њ,CaOЊІће÷–Ca2+µƒ≈дќї эќ™°°°°°°°°°£„ољьµƒCa‘≠„”ЇЌO‘≠„”µƒЇЋЉдЊаќ™a cm,‘тCaOЊІће√№ґ»µƒЉ∆Ћг љќ™°° °£
Ґс.(1)ҐуA°°
(2)ҐфA°°b
Ґт.(1)1s22s22p63s23p63d3
(2) °°C
°°C
(3)6°°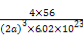
°Њљвќц°њҐс.(1)Ќ®єэЉ„ЌЉЈ÷ќц,MµƒµЏ“ї°ҐµЏґю°ҐµЏ»эµзјлƒ№±дїѓ≤їіу,Ћµ√ч»Ё“„ І»•„оЌв≤гµƒ3Єцµз„”,µЏЋƒµзјлƒ№ э÷µљѕіу,є Mќї”Џ÷№∆Џ±нµƒҐуA„е°£(2)Ќ®єэ““ЌЉ«вїѓќпЈ–µгЌЉѕс,a°Ґb÷–ѕаґ‘Ј÷„”÷ Ѕњ–°µƒ‘™ЋЎ«вїѓќпЈ–µг»ііу,“тќ™∆д–ќ≥…ЅЋ«вЉь,”¶ ф”ЏҐхA°ҐҐцA°ҐҐчA„е,÷ї”–µЏҐфA„е«вїѓќпЈ–µг“јіќ”…µЌµљЄя,cњ…“‘±н ЊµЏҐфA„е‘™ЋЎ°£
Ґт.(3)CaOЊІће÷–√њЄцCa2+ЌвќІ”–6ЄцO2-,Ca2+µƒ≈дќї эќ™6°£
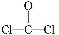
CaOЊІ∞ы÷–Їђ”–CaOЄц эќ™8°Ѕ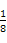 +6°Ѕ
+6°Ѕ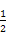 =4,∆д√№ґ»Љ∆Ћг љќ™:¶—=
=4,∆д√№ґ»Љ∆Ћг љќ™:¶—=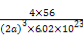 °£
°£