��Ŀ����
7��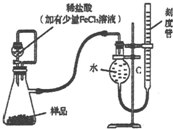 ������þMgO2������ϡ�ᣬ�����������������⣬��ҽѧ�Ͽ���Ϊ������ȣ�������þ��Ʒ�г���������MgO��ʵ���ҿ�ͨ�����ַ����ⶨ��Ʒ������þ�ĺ�����
������þMgO2������ϡ�ᣬ�����������������⣬��ҽѧ�Ͽ���Ϊ������ȣ�������þ��Ʒ�г���������MgO��ʵ���ҿ�ͨ�����ַ����ⶨ��Ʒ������þ�ĺ�������1��ij�о�С��������ͼװ�òⶨһ����������Ʒ�й�����þ�ĺ�����
��ϡ�����м�������FeCl3��Һ���������������������H2O2�ķֽ⣩��MgO2�ĵ���ʽΪ
 ��
���ڷ�Һ©���϶����ӵ���Ƥ�ܵ�������������
һ��ʹ��Һ©���е���Һ˳�����£�
��������������Һ��������������������Ӱ�죮
��2��ʵ���һ���ͨ���������ַ����ⶨ��Ʒ�й�����þ�ĺ�����
����I��ȡmg��Ʒ����������ϡ���ᣬ��ַ�Ӧ���ټ���NaOH��Һ��Mg2+������ȫ�����ˡ�ϴ�Ӻ�����������գ����յõ�ng���壮
����֪������Ksp[Mg��OH��2]=1.0��10-11��Ϊʹ����I��Mg2+��ȫ����[����Һ��c ��Mg2+����10-5mol•L-1]����Һ��pH����Ӧ����11������I�й�����þ����������Ϊ$\frac{7��m-n��}{2m}$���ú�m��n�ı���ʽ��ʾ����
����II����ȡ0.56g��Ʒ���ڵ���ƿ�У�����KI��Һ���������ᣬҡ�Ⱥ��ڰ�������5min��Ȼ����0.1mol•L-11Na2S2O3��Һ�ζ����ζ����յ�ʱ������VmL Na2S2O3��Һ��
����֪��I2+2Na2S2O3=Na2S4O6+2NaI��
�ڷ���II�м���0.1mol•L-1KI��Һ���������Ϊ200mL���ζ�ǰ���������������Һ��ָʾ������Ʒ�й�����þ����������Ϊ0.005V���ú�V�ı���ʽ��ʾ����
���� ��1����ͨ������˫��ˮ�ֽ����ɵ��������ɼ�������þ�ĺ��������Ȼ������������ӿ�˫��ˮ�ķֽ⣬�ӿ췴Ӧ���ʣ�������þ�����ӻ�����ݴ�д������ʽ��
�������������¶Ⱥ�ѹǿӰ���Һ©���϶����ӵ���Ƥ�ܵ������DZ��ֺ�ѹ���ú�ѹ��Һ©�����ŵ㻹������������Һ��������������������Ӱ�죬ʹ��Һ©���е���Һ˳�����£�
��2������I����������þ���ܶȻ����������Һ�����������ӵ�Ũ�ȣ������Һ��PH������n������þ������m�ǹ�����þ������þ��������������������ʵ�������ʽ������������ʵ����������ݹ�����þ�����ʵ��������������������
���������0.1mol•L-1KI��Һ��Ҫʹ������þ��ȫ��Ӧ�����ݵ����غ��֪��ϵʽMgO2��2KI���ݴ˼���KI��Һ����������ڵ�����������ɫ����������������Һ��ָʾ�������ݵ����غ��ҳ���ϵʽMgO2��2KI��I2��2Na2S2O3�������������þ������������
��� �⣺��1����ʵ��װ�����Ӻ��Ժ�ʵ��ǰ����еIJ����Ǽ��װ�õ������ԣ�������þ����ˮ����˫��ˮ��˫��ˮ�ֽ⣬ͨ������˫��ˮ�ֽ����ɵ��������ɼ�������þ�ĺ��������Ȼ������������ӿ�˫��ˮ�ķֽ⣬�ӿ췴Ӧ���ʣ�������þ�����ӻ�����ݴ�д������ʽΪ ��
��
�ʴ�Ϊ���������������H2O2�ķֽ⣩�� ��
��
�����������������¶Ⱥ�ѹǿӰ��������ú�ѹ��Һ©�����ŵ㻹������������Һ��������������������Ӱ�죬ʹ��Һ©���е���Һ˳�����£�
�ʴ�Ϊ������������Һ��������������������Ӱ�죻
��2������I����������þ���ܶȻ�������֪������Һ��c��Mg2+��=l��10-5mol/Lʱ��Ksp[Mg��OH��2]=1��10-11=c��Mg2+��•c2��OH-������Һ��OH-Ũ�ȵ���1��10-3mol/L��������Һ��pH=11���������þ�����ʵ�����xmol������þ���ʵ�����ymol����56x+40y=m����x+y����40=n�����x=$\frac{m-n}{16}$������I�й�����þ����������Ϊ��$\frac{\frac{m-n}{16}��56}{m}$=$\frac{7��m-n��}{2m}$��
�ʴ��ǣ�11��$\frac{7��m-n��}{2m}$��
������0.56g��Ʒ���ڵ���ƿ�У�������Ʒ�ж���MgO2���������ʵ���Ϊ0.01mol������0.1mol•L-1KI��Һ��Ҫʹ������þ��ȫ��Ӧ�����ݵ����غ��֪��ϵʽMgO2��2KI������KI��Һ�����Ϊ$\frac{0.01mol��2}{0.1mol•{L}^{-1}}$=0.2L=200mL�����ڵ�����������ɫ����������������Һ��ָʾ������0.1mol•L-11Na2S2O3��Һ�ζ����ζ����յ�ʱ������VmL��������ʵ���ΪV��10-4mol�����ݵ����غ��ҳ���ϵʽMgO2��2KI��I2��2Na2S2O3�������������þ�����ʵ���Ϊ$\frac{1}{2}$��V��10-4mol�����Թ�����þ����������Ϊ$\frac{\frac{1}{2}��V��1{0}^{-4}��56}{0.56}$=0.005V��
�ʴ�Ϊ��200��������Һ��0.005V��
���� �����Ǹ߿��еij������ͣ������е��Ѷȵ����⣬�����ۺ���ǿ�����ض�ѧ��������������ѵ��������������ѧ���淶�Ͻ���ʵ����ơ�����������


| A�� | ͼ4Ϊ��Ũ������Һ��ͭ��Ӧ�Ʊ����ռ�����NO2 | |
| B�� | ͼ2Ϊ�Ʊ��������� | |
| C�� | ͼ3Ϊ����һ�����ʵ���Ũ�ȵ�������Һ | |
| D�� | ͼ1Ϊ֤���ǽ�����ǿ����S��C��Si |
 ��ͼ����ʾ��Ӧ��mA��g��+nB��g��?pC��g��+qD��g������H���ڲ�ͬ�¶��¾���һ��ʱ����������ϵ��C�İٷֺ������¶�T�Ĺ�ϵ��ͼ����ʾ��һ�������´ﵽƽ���tʱ�̸ı�ѹǿ�����½���ƽ��ķ�Ӧ���̣��ɴ˿��жϸ÷�Ӧ�У�������
��ͼ����ʾ��Ӧ��mA��g��+nB��g��?pC��g��+qD��g������H���ڲ�ͬ�¶��¾���һ��ʱ����������ϵ��C�İٷֺ������¶�T�Ĺ�ϵ��ͼ����ʾ��һ�������´ﵽƽ���tʱ�̸ı�ѹǿ�����½���ƽ��ķ�Ӧ���̣��ɴ˿��жϸ÷�Ӧ�У�������| A�� | m+n��p+q����H��0 | B�� | m+n��p+q����H��0 | C�� | m+n��p+q����H��0 | D�� | m+n��p+q����H��0 |
| A�� | ����0.3 mol Cl2ʱ��ת�Ƶ��ӵ����ʵ���Ϊ0.6 mol | |
| B�� | �÷�Ӧ�У����ɵ�Cl2��Ħ������ԼΪ70.7 g/mol | |
| C�� | ��0.1 mol K37ClO3������H35Cl�����ʵ���Ϊ0.6 mol | |
| D�� | �÷�Ӧ�У�H35Cl����������Ӧ��KCl�ǻ�ԭ���� |
| A�� | ȼ�ճס�����ƿ | B�� | �������Թ� | C�� | �������ձ� | D�� | ��ƿ���ձ� |
| A�� | SO2��O2��H2S | B�� | H2��O2��F2 | C�� | O2��C12��HBr | D�� | H2S��CO2��H2 |
| A�� | ������ | B�� | ̼������ | C�� | ������� | D�� | �Ȼ��� |