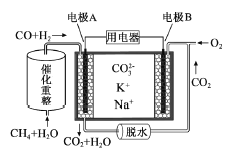
��Ŀ����
����Ŀ���������洦����Ƥ�����ơ�ӡȾ�ȶ�������ɸ���Ⱦ�����۸������۸����Ըߣ����ױ����������������������
����ҵ�ϴ������Ժ�Cr2O72����ˮ�ķ������£�
����Cr2O72�������Է�ˮ�м���FeSO4��Һ��ʹCr2O72��ȫ��ת��ΪCr3����д���÷�Ӧ�����ӷ���ʽ��_____��
��������Һ��pH��ʹCr3����ȫ������ʵ���Ҵ��Բⶨ��ҺpH�ķ���Ϊ_____��25������������Һ��pH=8������Һ�в���Cr3�������ʵ���Ũ��Ϊ_____mol/L������֪25��ʱ��Ksp[Cr(OH)3]=6.3��10��31��
����Ԫ����Ũ�ȵIJⶨ��ȷ��ȡ25.00mL��Cr2O72����Cr3�������Է�ˮ�������м���������(NH4)2S2O8��Һ��Cr3��������Cr2O72������г�ȥ������(NH4)2S2O8����������Һ�м��������KI��Һ����ַ�Ӧ���Ե���Ϊָʾ���������еμ�0.015mol/L��Na2S2O3����Һ���յ�ʱ����Na2S2O3��Һ20.00mL��
��֪�ⶨ�����з����ķ�Ӧ���£�
��2Cr3����3S2O82����7H2O =Cr2O72����6SO42����14H��
��Cr2O72����6I����14H��=2Cr3����3I2��7H2O
��I2��2S2O32��=2I����S4O62��
�����ˮ�и�Ԫ����Ũ�ȣ���λ��mg��L��1��д��������̣���_____________________
���𰸡�Cr2O72����6Fe2����14H��=2Cr3����6Fe3����7H2O ��pH��ֽ���ڽྻ�ı������ϣ��ò�����պȡ��Һ�� ����pH��ֽ�ϣ��������ɫ������ 6.3��10��13 �ɷ���ʽ��֪��Cr~3Na2S2O3��֪��n(Na2S2O3)=20.00mL��0.015mol/L=3��10��4mol
n(Cr)=1��10��4mol��m(Cr)=1��10��4mol��52g��mol��1=5.2��10��3 g=5.2mg����ˮ�и�Ԫ����Ũ��=![]() =208 mg��L��1��
=208 mg��L��1��
��������
��1����Cr2O72����Fe2��֮�������Ի����·���������ԭ��Ӧ��
������ʹ��pH��ֽ������Һ����Է������з�����
ʵ���Ҵ��Բⶨ��ҺpH�ķ�����ʹ��pH��ֽ����pH��ֽ���ڽྻ�ı������ϣ��ò�����պȡ��Һ������pH��ֽ�ϣ��������ɫ�����գ�����c��Cr3����= 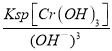 ���м��㣻
���м��㣻
��2���ɷ���ʽȷ����ϵʽ��Cr~3Na2S2O3��֪��n(Cr)= ![]() n(Na2S2O3)�����������m(Cr) mg����ˮ�и�Ԫ����Ũ��=
n(Na2S2O3)�����������m(Cr) mg����ˮ�и�Ԫ����Ũ��= ![]() ��
��
��1��������������ԭ��Ӧ���������ӱ�����Ϊ�����ӣ�Cr2O72����6Fe2����14H��=2Cr3����6Fe3����7H2O��
��ʵ���Ҵ��Բⶨ��ҺpH�ķ�����ʹ��pH��ֽ����pH��ֽ���ڽྻ�ı������ϣ��ò�����պȡ��Һ������pH��ֽ�ϣ��������ɫ�����գ�����Һ�в���Cr3�������ʵ���Ũ��Ϊ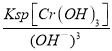 =
=![]() =6.3��10��13��
=6.3��10��13��
��2���ɷ���ʽȷ����ϵʽ��Cr~3Na2S2O3��֪��n(Na2S2O3)=20.00mL��0.015mol/L=3��10��4mol��n(Cr)=1��10��4mol��m(Cr)=1��10��4mol��52g��mol��1=5.2��10��3 g=5.2mg����ˮ�и�Ԫ����Ũ��=![]() =208 mg��L��1��
=208 mg��L��1��

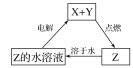
 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�