��Ŀ����
����Ŀ����֪��Ȳ�뱽������ȫȼ�յ��Ȼ�ѧ����ʽ������ʾ��
��2C2H2��g��+5O2��g����4CO2��g��+2H2O��l��+2600kJ
��2C6H6��g��+15O2��g����12CO2��g��+6H2O��l��+6590kJ
����˵����ȷ����
A.2mol C2H2��g�� ��ȫȼ��������̬ˮʱ���ȴ���2600kJ
B.2mol C6H6��l�� ��ȫȼ������Һ̬ˮʱ���ȴ���6590kJ
C.��ͬ�����£���������C2H2��g����C6H6��g����ȫȼ�գ�C6H6��g�����ȸ���
D.C2H2��g�� ��������C6H6��g�� �Ĺ������ڷ��ȷ�Ӧ
���𰸡�D
��������
A��2C2H2(g)+5O2(g)��4CO2(g)+2H2O(l)+2600kJ��2mol C2H2(g)��ȫȼ��������̬ˮʱ����С��2600kJ����A����
B��2C6H6(g)+15O2(g)��12CO2(g)+6H2O(l)+6590kJ��2molC6H6(l)��ȫȼ������Һ̬ˮʱ���ȵ���6590kJ����B����
C����ͬ�����£���������C2H2(g)��C6H6(g)��ȫȼ�գ�1gC2H2(g)���ʵ���=![]() mol��1gC6H6(g)���ʵ���=
mol��1gC6H6(g)���ʵ���=![]() mol��C2H2(g)��ȫȼ�շ���
mol��C2H2(g)��ȫȼ�շ���![]() =50kJ��C6H6(g)��ȫȼ�շ���
=50kJ��C6H6(g)��ȫȼ�շ���![]() =42.2kJ��C2H2(g)��ȫȼ�շ��ȸ��࣬��C����
=42.2kJ��C2H2(g)��ȫȼ�շ��ȸ��࣬��C����
D���ɷ�Ӧ����3-��Ӧ�ڵã�6C2H2(g)=2C6H6(g)+1210kJ�����C2H2(g)��������C6H6(g)�Ĺ������ڷ��ȷ�Ӧ����D��ȷ��
�ʴ�Ϊ��D��

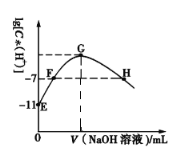
����Ŀ��ij̽��С�����ñ�ͪ�������Ӧ(CH3COCH3��Br2![]() CH3COCH2Br��HBr)���о���Ӧ��Ũ���뷴Ӧ���ʵĹ�ϵ����Ӧ���� v(Br2) ͨ���ⶨ�����ɫ��ʧ�����ʱ����ȷ������һ���¶��£��������ʵ�����ݣ�
CH3COCH2Br��HBr)���о���Ӧ��Ũ���뷴Ӧ���ʵĹ�ϵ����Ӧ���� v(Br2) ͨ���ⶨ�����ɫ��ʧ�����ʱ����ȷ������һ���¶��£��������ʵ�����ݣ�
ʵ����� | ��ʼŨ��c/mol��L��1 | ����ɫ��ʧ����ʱ��t/s | ||
CH3COCH3 | HCl | Br2 | ||
�� | 0.80 | 0.20 | 0.0010 | 290 |
�� | 1.60 | 0.20 | 0.0010 | 145 |
�� | 0.80 | 0.40 | 0.0010 | 145 |
�� | 0.80 | 0.20 | 0.0020 | 580 |
����ʵ���������ó��Ľ�������ȷ����(����)
A������c(CH3COCH3)��v(Br2)����
B��ʵ��ں͢۵�v(Br2)���
C������c(HCl)��v(Br2)����
D������c(Br2)��v(Br2)����
����Ŀ���������N2��O2����������ֱ�Ϊ0.8��0.2���ڷ���¯�н��������ÿ����г�����գ� 4FeS2+11O2![]() 2Fe2O3+8SO2
2Fe2O3+8SO2
(1)����������SO2���������������_______%������һλС������
(2)���ӽӴ��ҳ�����������SO2��O2��N2��SO3�������Ϊ0.5��1��20��x����x=_______����ʱ��Ӧ��SO2��ת����Ϊ_______%������һλС������
(3)��ʵ�������У��ڽӴ���SO2��O2���������1�U4��Ϊ���ˡ�������¯ͨ��Ӵ��ҵ�¯����SO2��O2��N2�������Ϊ8��1��48��������Ϊ2.5m3/s, �������Ӵ�����ͨ��Ŀ���������ӦΪ_______m3/s������һλС�������������ͬ��ͬѹ�²ⶨ����
(4)��������������������һ������ˮ���գ��õ�����β�Ʒ��(NH4)2SO3��NH4HSO3�Ļ���ȡ��ͬ��������Σ��ֱ����x mol/L��50.00mL NaOH��Һ�У�����ˮԡ�����¶�������������ֽ⣩����ʹ����ȫ���ݳ����������Ũ������ȫ���ա�ʵ���������£�
ʵ����� | �������(g) | Ũ�������ӵ�����(g) |
��һ�� | 1.570 | 0.340 |
�ڶ��� | 3.140 | 0.680 |
������ | 4.710 | 0.510 |
���Ĵ� | 6.280 | 0 |
�ٸ������(NH4)2SO3��NH4HSO3�����ʵ���֮��Ϊ___________��
������x��ֵ��________________